
DIETHYL IODOMETHYLPHOSPHONATE synthesis
- Product Name:DIETHYL IODOMETHYLPHOSPHONATE
- CAS Number:10419-77-9
- Molecular formula:C5H12IO3P
- Molecular Weight:278.03
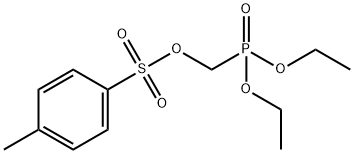
31618-90-3
428 suppliers
$5.00/5g

10419-77-9
57 suppliers
$61.00/1g
Yield: 91%
Reaction Conditions:
with sodium iodide in acetoneReflux;
Steps:
O,O-Diethyl iodomethylphosphonate (7)
To a stirred solution of tosylate 5 (644 mg, 2 mmol, 1.00 equiv) in acetone (10 mL) was added sodium iodide (600 mg, 4 mmol, 2.00 equiv) in a single portion. The resulting suspension was stirred at reflux overnight and subsequently allowed to cool. Water (20 mL) was added to the reaction mixture and the product was extracted into dichloromethane (20 mL) and washed with 2% sodium thiosulfate (10 mL). Drying of the solvent over magnesium sulfate and removal of the solvent in vacuo furnished the title compound as a pale yellow oil (485 mg, 1.82 mmol, 91%). 1H NMR (400 MHz, CDCl3): 1.30 (6H, t, J = 7.20 Hz, OCH2CH3), 2.99 (2H, d, J = 10.37 Hz, ICH2), 4.12 (4H, overlapping doublet of quartets appearing as a quintet, JH-P = JH-H = 7.40 Hz, OCH2). 13C NMR (100 MHz, CDCl3): -14.32 (d, JC-P = 155.00 Hz, CH2I), 16.33 (d, JC-P = 5.98 Hz, CH3), 63.34 (d, JC-P = 6.44 Hz, OCH2) 31P NMR (175 MHz, CDCl3): 20.63. HRMS (ESI+): Exact mass calc. C7H17IO4P [M+H] = 322.9904; Found = 322.9909.
References:
Jones, David J.;O’Leary, Eileen M.;O’Sullivan, Timothy P. [Beilstein Journal of Organic Chemistry,2019,vol. 15,p. 801 - 810] Location in patent:supporting information
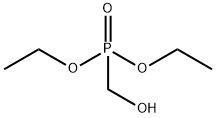
3084-40-0
240 suppliers
$10.00/10g

10419-77-9
57 suppliers
$61.00/1g

75-11-6
343 suppliers
$10.00/5g
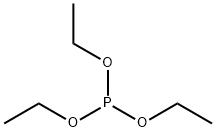
122-52-1
248 suppliers
$9.00/5g

10419-77-9
57 suppliers
$61.00/1g

1660-94-2
180 suppliers
$10.00/1g
