DIETHYL PHENYLPHOSPHONATE synthesis
- Product Name:DIETHYL PHENYLPHOSPHONATE
- CAS Number:1754-49-0
- Molecular formula:C10H15O3P
- Molecular Weight:214.2
Yield: 89%
Reaction Conditions:
with bis(1,5-cyclooctadiene)nickel (0);2-(di-tert-butylphosphaneyl)-4-methoxy-N,N-dimethylaniline;N-ethyl-N,N-diisopropylamine in toluene at 110; for 24 h;Inert atmosphere;Schlenk technique;
Steps:
Ni-Catalyzed cross-coupling of aryl tosylates with H(O)P(OR)2.
General procedure: Under the atmosphere of N2, 0.2 mmol of aryl tosylate 1a, 3 mol % Ni(cod)2, 3 mol % L8, DIPEA (0.6 mmol), and 2 mL of toluene were charged into a 10 mL Schlenk tube, then 0.15 mmol of (i-PrO)2P(O)H (2a) was added to the mixture. The reaction was processed at 110°C for 10 h, then the other 0.15 mmol of (i-PrO)2P(O)H (2a) was added. The mixture was further stirred at 110°C for 14 h. After removal of the volatiles, the residue was passed through a short silica chromatographical column to afford analytically pure organophosphorus compound 3a. Most of thus synthesized products had been reported earlier, and their spectral data matched those presented in literature. The products 3e, 3f, 3n, 3o, 3p', 3r, and 3r' were the newly synthesized compounds.
References:
Li, Chun-jing [Russian Journal of General Chemistry,2020,vol. 90,# 4,p. 725 - 730]

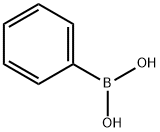
88284-48-4
162 suppliers
$9.00/250mg
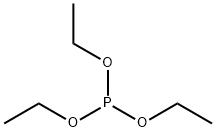
122-52-1
246 suppliers
$9.00/5g

1754-49-0
79 suppliers
$42.00/5g
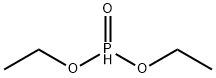
762-04-9
318 suppliers
$10.00/10 g
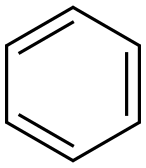
71-43-2
671 suppliers
$11.19/25ML

1754-49-0
79 suppliers
$42.00/5g

88284-48-4
162 suppliers
$9.00/250mg

762-04-9
318 suppliers
$10.00/10 g

1754-49-0
79 suppliers
$42.00/5g