
Diisobutylcarbinol synthesis
- Product Name:Diisobutylcarbinol
- CAS Number:108-82-7
- Molecular formula:C9H20O
- Molecular Weight:144.25

108-83-8
317 suppliers
$16.00/25mL

1072-05-5
27 suppliers
$455.00/1g

108-82-7
165 suppliers
$12.00/5g
Yield:-
Reaction Conditions:
with hydrogen at 60; under 750.075 Torr; for 3 h;
Steps:
2.3. Catalyst testing
General procedure: The hydrogenation of ketones was carried out in the gas phase in flowing H2. The catalysts were tested at 60-100 °C under atmospheric pressure in a Pyrex fixed-bed down-flow reactor (9 mm internal diameter) fitted with an online gas chromatograph (Varian Star 3400 CX instrument with a 30 m × 0.25 mm HP INNOWAXcapillary column and a flame ionisation detector). The temperature in the reactor was controlled by a Eurotherm controller using a thermocouple placed at the top of the catalyst bed. The gas feed contained a variable amount ketone in H2 as a carrier gas. The ketone was fed by passing H2 carrier gas flow controlled by a Brooks mass flow controller through a stainless steel saturator, which held the liquid ketone at appropriate temperature to maintain the chosen reactant partial pressure. The downstream gas lines and valves were heated to 180°C to prevent substrate and product condensation. The gas feed entered the reactor at the top at a flow rate of 20-100 mL min-1. The reactor was packed with 0.2 g catalyst powder of 45-180 m particle size. In some cases, to reduce conversion, a smaller amount of catalyst was used as a homogeneous mixture with silica of a total weight of 0.2 g. Prior to reaction, the catalysts were pretreated in H2 for 1 h at the reaction temperature unless stated otherwise. The dehydration of 2-methyl-4-pentanol was studied similarly, except using N2 as acarrier gas instead of H2. Once reaction started, the downstream gas flow was analysed by the online GC to obtain reactant conversion and product selectivity. The selectivity was defined as moles of product formed per one mole of reactant converted and quoted in mole per cent. The mean absolute percentage error in conversion and selectivity was ≤10% and the carbon balance was maintained within 95%.
References:
Alharbi;Kozhevnikova;Kozhevnikov [Applied Catalysis A: General,2015,vol. 504,p. 457 - 462]

108-83-8
317 suppliers
$16.00/25mL

108-82-7
165 suppliers
$12.00/5g
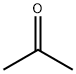
67-64-1
0 suppliers
$17.30/10ml
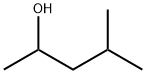
108-11-2
381 suppliers
$14.00/25mL

187737-37-7
1 suppliers
inquiry

74-98-6
127 suppliers
$20.00/1ea
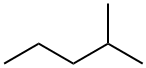
107-83-5
244 suppliers
$10.00/1g

108-82-7
165 suppliers
$12.00/5g

108-83-8
317 suppliers
$16.00/25mL
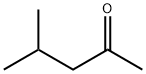
108-10-1
907 suppliers
$12.00/25ml
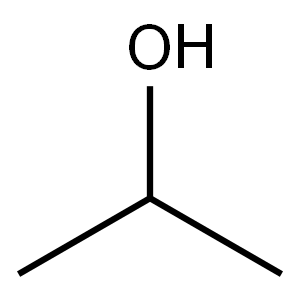
67-63-0
1641 suppliers
$16.00/25ML

108-67-8
386 suppliers
$5.00/5 g

504-20-1
169 suppliers
$10.00/250mg

108-82-7
165 suppliers
$12.00/5g

504-20-1
169 suppliers
$10.00/250mg

108-82-7
165 suppliers
$12.00/5g