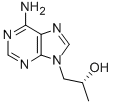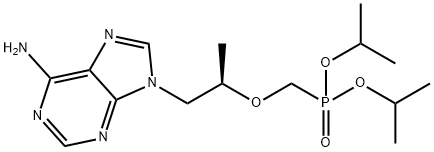
Diisopropyl Tenofovir FuMarate synthesis
- Product Name:Diisopropyl Tenofovir FuMarate
- CAS Number:160616-04-6
- Molecular formula:C15H26N5O4P
- Molecular Weight:371.37
Yield:160616-04-6 91%
Reaction Conditions:
with magnesium 2-methylpropan-2-olate in water;N,N-dimethyl-formamide at -15 - 20; for 44 h;Inert atmosphere;
Steps:
2 Example 2: Synthesis of diesters of (R)-9-[2-(phosphonomethoxy)propyl]adenine
The Buchi reactor (10 L) was preheated to 150 °C and traces of moisture were blown away with the nitrogen stream. The reactor was filled with argon and cooled down to RT, dry DMF (5 L, ~ 20 ppm of H2O, distilled from P2O5) was added and stirring was switched on (200 rev/min) at RT. Starting compound 1 (111.3 g, 0.576 mol) and tosylate 2b (222.1 g, 0.634 mol) were added. When the starting material was dissolved at RT, the reaction mixture was cooled down to -10 °C. Then the reaction mixture was cooled to -15 °C and (tBuO)2Mg (147.3, 0.864 mol) was started to be added continuously. White suspension was formed during the addition of (tBuO)2Mg at -10 °C, which was continuously consumed by the reaction. The reaction was monitored by TLC. The reaction was completed in ~ 44 h. Then H2O (200 mL) was added to stop the reaction (and to destroy unreacted tosylate). The reaction mixture was slowly warmed up to RT and solvents were removed at 60 °C in vacuo. The residue was dissolved in H2O (1 L) and the solution was placed into a continuous extractor and was extracted with CH2Cl2 (TLC showed none product in the water phase). The organics were dried (MgSO4) and evaporated at 30 °C in vacuo. Hexane (200 mL) was added to the thick oily residue, this mixture was sonicated for 20 min, and hexane phase was removed (impurities soluble in hexane can be removed by this procedure). EtOAc (150 mL) was added to the residue and the mixture was sonicated until all product crystallized. After cooling in ice bath, the crystals were filtered off, washed (2 x 100 mL of mixture hexane/EtOAc, 5 °C), and dried at 60°C for 4 days (under vacuum with P2O5) to give 115.5 g (54%) of 3b (93% purity). The yield were subsequently improved to up to 91 %.
References:
WO2015/51874,2015,A1 Location in patent:Page/Page column 21; 22; 6

116-17-6
174 suppliers
$14.00/5mL

160616-04-6
13 suppliers
inquiry

35717-98-7
32 suppliers
inquiry

160616-04-6
13 suppliers
inquiry

138247-57-1
1 suppliers
inquiry

160616-04-6
13 suppliers
inquiry