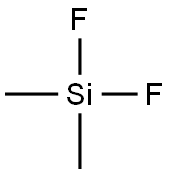
DIMETHYLDIFLUOROSILANE synthesis
- Product Name:DIMETHYLDIFLUOROSILANE
- CAS Number:353-66-2
- Molecular formula:C2H6F2Si
- Molecular Weight:96.15
1009-93-4
145 suppliers
$10.00/1g
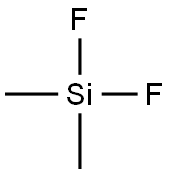
353-66-2
40 suppliers
inquiry
Yield: 92%
Reaction Conditions:
with boron trifluoride diethyl etherate in neat (no solvent) at 20 - 120; for 3 h;Temperature;
Steps:
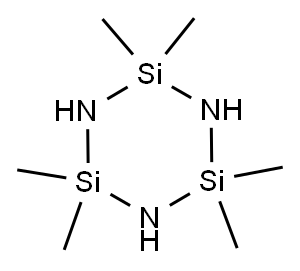
Procedure for the depolymerization of 1,1,3,3,5,5-hexamethylcyclotrisilazane 5
A ask was charged withcompound 5 (4.6 mmol) and boron triuoride diethyl etherate2 (3.0 equiv., 13.8 mmol, based on the subunit). A Vigreuxcolumn and a distillation head were connected. The mixturewas stirred and heated to 100°C (oil bath). The formed diuorodimethylsilane (bp = 16°C24) was continuously distilledo and collected. After 3 h the yield was determined and thequality was analyzed by NMR. Diuorodimethylsilane 5: 1HNMR: δ = 0.31 (t, 3JHF = 6.2 Hz) ppm. 13C{1H} NMR: δ =-3.45 (t, 2JCF = 16.7 Hz) ppm. 19F NMR: δ = -131.4 (sept, 3JHF= 6.2 Hz, 1JSiF = 289.9 Hz) ppm. 29Si{1H} NMR: δ = -2.4 ppm.Diethyl ether: 1H NMR: δ = 3.69 (q, 3JHH = 7.0 Hz, CH2), 1.26(t, 3JHH = 7.0 Hz, CH3) ppm. 13C{1H} NMR: δ = 67.0 (CH2),14.7 (CH3) ppm
References:
Döhlert, Peter;Pfrommer, Johannes;Enthaler, Stephan [Phosphorus, Sulfur and Silicon and the Related Elements,2016,vol. 191,# 9,p. 1189 - 1193]
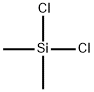
75-78-5
363 suppliers
$15.00/5 g
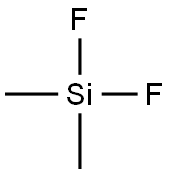
353-66-2
40 suppliers
inquiry
3277-26-7
356 suppliers
$13.00/5 g
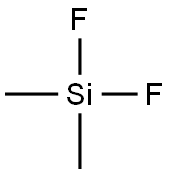
353-66-2
40 suppliers
inquiry

![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)