
Ethyl 2-aminopropanoate hydrochloride synthesis
- Product Name:Ethyl 2-aminopropanoate hydrochloride
- CAS Number:617-27-6
- Molecular formula:C5H12ClNO2
- Molecular Weight:153.61
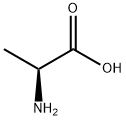
302-72-7
619 suppliers
$8.00/100g

617-27-6
189 suppliers
$10.00/5g
Yield: 91%
Reaction Conditions:
in ethanol
Steps:
II N-Chloroacetyl alanine ethyl ester
N-Chloroacetyl alanine ethyl ester The procedure of C. S. Marvel and W. A. Noyes, J. Amer. Chem. Soc., 42, 2265 (1920) was followed. Thirty grams (0.34 mole) of alanine was suspended in 150 ml of ethyl alcohol in a reaction flask and anhydrous hydrogen chloride gas was passed into the mixture at an initial temperature of 25° C. Addition was continued until 50 g (1.4 moles) of gas had been added. The temperature rose to 65° C. over the course of the addition. After addition was complete, the mixture was heated at reflux for 3.5 hours. During this time there was formation of a solid. Upon cooling, the solid separated and was removed by filtration to give 47.5 g of alanine ethyl ester hydrochloride (91% yield).
References:
Stauffer Chemical Company US4361439, 1982, A
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

617-27-6
189 suppliers
$10.00/5g

71754-74-0
1 suppliers
inquiry

617-27-6
189 suppliers
$10.00/5g


