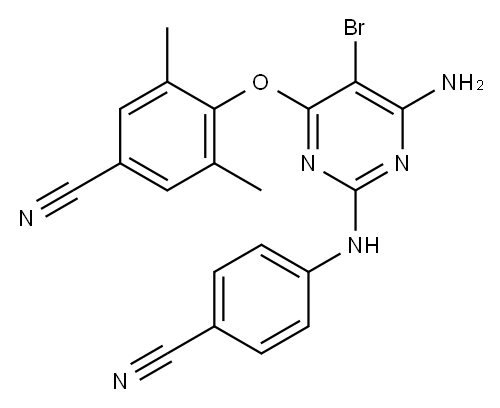
Etravirine synthesis
- Product Name:Etravirine
- CAS Number:269055-15-4
- Molecular formula:C20H15BrN6O
- Molecular Weight:435.28

Process for the preparation of 4- [6-amino-5-bromo-2- [(4-cyanophenyl) amino]-4-pyrimidinyl]oxy]-3.5-dimethylbe nzonitrile (Etravirine); Rajan, Srinivasan Thirumalai; Eswaraiah, Sajja; Gutta, Madhusudhan; Peri, Seetha Rama Sarma; Assignee MSN Laboratories Private Limited, India; 2016; Patent Information; Apr 21, 2016; WO 2016059646 A2

269055-76-7
24 suppliers
inquiry

269055-15-4
269 suppliers
$5.00/1mg
Yield:269055-15-4 40.5%
Reaction Conditions:
in water;
Steps:
A.19 EXAMPLE A19
EXAMPLE A19 4-[[5-bromo4-(4-cyano-2,6-dimethylphenoxy)-6-chloro-2-pyrimidinyl]amino]benzonitrile (0.00250 mol) and NH3/1,4-dioxane 0.5M (0.015 mol) were heated in a pressure vessel at 150° C. for 4 days. The sample was allowed to sit at ambient conditions for 2 days. Water was added slowly to the mixture until a precipitate formed. The mixture was stirred for 2 hours and filtered. The solid was recrystallized from CH3CN to obtain 0.58 g (fraction 1). The filtrate was evaporated (fraction 2). Both fractions were combined and purified by column chromatography, eluding with CH2Cl2. The resulting residue of the desired fraction was recrystallized from CH3CN to yield 0.44 g of 4-[[4-amino-5-bromo-6-(4-cyano-2,6-dimethylphenyloxy)-2-pyrimidinyl]amino]benzonitrile (40.5%).
References:
US2003/186990,2003,A1

1030633-38-5
4 suppliers
inquiry

269055-15-4
269 suppliers
$5.00/1mg

1415796-11-0
3 suppliers
inquiry
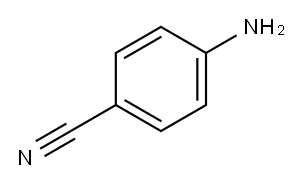
873-74-5
616 suppliers
$9.00/1g

269055-15-4
269 suppliers
$5.00/1mg

101012-11-7
29 suppliers
inquiry

4198-90-7
244 suppliers
$6.00/5g

873-74-5
616 suppliers
$9.00/1g

269055-15-4
269 suppliers
$5.00/1mg

5637-42-3
121 suppliers
$7.00/1g

269055-15-4
269 suppliers
$5.00/1mg








