
Hypophosphorous acid synthesis
- Product Name:Hypophosphorous acid
- CAS Number:6303-21-5
- Molecular formula:HO2P
- Molecular Weight:63.9805
1. Boiling white phosphorus with calcium hydroxide:
P4 + 4Ca(OH)2 + 8H2O → 4Ca(H2PO2)2 + 4H2
The calcium salt is soluble in water. Treatment with sulfuric acid yields thehypophosphorous acid:
(H2PO2)2Ca + H2SO4 → 2H3PO2 + CaSO4
The product mixture is filtered to remove insoluble CaSO4. The aqueous solu-tion of hypophosphorous acid is concentrated under reduced pressure.Concentrated baryta water may be used instead of calcium hydroxide.2. By treating sodium hypophosphite, NaH2PO2with an ion-exchange resin.The sodium salt may be produced by boiling white phosphorus with a solutionof sodium hydroxide, a reaction similar to (1) above.
PH3 + 2I2 + 2H2O → H3PO2 + 4HI
The above method may be considered safer than that involving heating whitephosphorus with an alkali.
Hypophosphorous acid must be stored below 50°C. It is sold commerciallyas an aqueous solution at various concentrations.
![Tetraphosphatricyclo[1.1.0.02,4]butane](/CAS/20200611/GIF/100320-09-0.gif)
100320-09-0
1 suppliers
inquiry
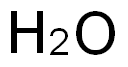
7732-18-5
458 suppliers
$13.50/100ML
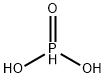
13598-36-2
315 suppliers
$25.00/10g

7803-51-2
3 suppliers
$1780.00/10g

6303-21-5
299 suppliers
$35.50/50g
Yield:-
Reaction Conditions:
with hydrogenchloride;cadmium in ethanol;Electrochemical reaction;Inert atmosphere;Schlenk technique;Reagent/catalyst;
Steps:
Electrochemical reduction of white phosphorus.
General procedure: A solution for electrolysis was prepared by suspending white phosphorus (70 mg, 0.56 mmol) in a water-ethanol mixture (30 mL) at 50 °C. In some experiments with the Al anode, a small amount of terephthalaldehyde (0.5 mmol) was added to assess the effect of organic mediator on the course of the electrochemical process (see Table 1). Then, 2 M aqueous HCl (0.5 ml) was added to the resulted finely divided suspension (opaque solution) and the direct current voltage with a current of 150 mA was applied. During the electrolysis, the solution became clear. The reaction mixture was analyzed 10 min after starting the electrolysis by 31P NMR spectroscopy and GC/MS. The conditions of electrochemical processes and the percentages of phosphorus products in the reaction mixture are given in Table 1. The 31P NMR spectral characteristics of the phosphorus products obtained correspond to those published earlier15.
References:
Yakhvarov;Gorbachuk;Khayarov, Kh. R.;Morozov;Rizvanov, I. Kh.;Sinyashin [Russian Chemical Bulletin,2014,vol. 63,# 11,p. 2423 - 2427][Izv. Akad. Nauk, Ser. Khim.,2014,# 11,p. 2423 - 2427,5]

7803-51-2
3 suppliers
$1780.00/10g

13598-36-2
315 suppliers
$25.00/10g

6303-21-5
299 suppliers
$35.50/50g

7803-51-2
3 suppliers
$1780.00/10g

6303-21-5
299 suppliers
$35.50/50g