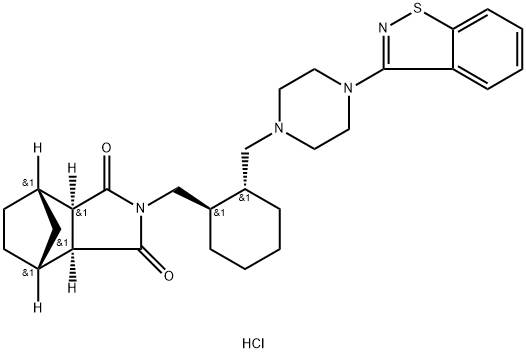
Lurasidone hydrochloride synthesis
- Product Name:Lurasidone hydrochloride
- CAS Number:367514-88-3
- Molecular formula:C28H37ClN4O2S
- Molecular Weight:529.14
![(3aR,7aR)-4'-(1,2-Benzisothiazol-3-yl)octahydrospiro[2H-isoindole-2,1'-piperaziniuM] Methanesulfonate](/CAS/20180906/GIF/186204-37-5.gif)
186204-37-5
92 suppliers
inquiry
14805-29-9
311 suppliers
$35.00/5g

367514-88-3
316 suppliers
$50.00/10mg
Yield:367514-88-3 98.3%
Reaction Conditions:
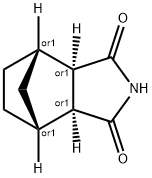
Stage #1: (3aR,7aR)-4’-(benzo[d]isothiazol-3-yl)octahydrospiro[isoindole-2,1’-piperazin]-2-ium methanesulfonate;(3aR,4S,7R,7aS)?4,7?methylene?1H?isoindole?1,3(2H)-dionewith potassium carbonate in toluene at 105; for 15 h;Industrial scale;
Stage #2: with hydrogenchloride in isopropyl alcohol;Industrial scale;
Steps:
8 industrial synthesis of Lurasidone
Intermediate 4 (28.8 kg, 66.2 mol), intermediate 5 (12.0 kg, 72.8 mol) andpotassium carbonate (11.0 kg, 79.7 mol) are suspended in toluene (270 1), and theresulting suspension is heated at 105°C for 15 hours, monitoring the reaction by UPLC. When the reaction is complete, the mixture is left to cool at room temperature, and water (90 1) is added. The phases are separated, the organic solution is concentrated to a small volume, and Lurasidone is isolated as thehydrochloride after treatment with HCI in isopropanol (34.4 kg, yield 98.3%, purity [HPLCJ 99.49%).
References:
WO2015/56205,2015,A1 Location in patent:Page/Page column 14

14166-28-0
92 suppliers
$69.00/250mg

367514-88-3
316 suppliers
$50.00/10mg

87691-87-0
402 suppliers
$10.00/1g

367514-88-3
316 suppliers
$50.00/10mg

14805-29-9
311 suppliers
$35.00/5g

367514-88-3
316 suppliers
$50.00/10mg

1421374-96-0
0 suppliers
inquiry

367514-88-3
316 suppliers
$50.00/10mg