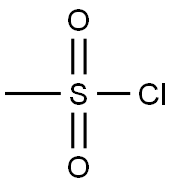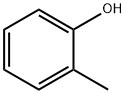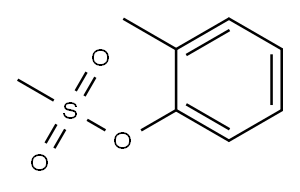
Methanesulfonic acid 2-methylphenyl ester synthesis
- Product Name:Methanesulfonic acid 2-methylphenyl ester
- CAS Number:1009-01-4
- Molecular formula:C8H10O3S
- Molecular Weight:186.23
Yield:1009-01-4 100%
Reaction Conditions:
with triethylamine in ethyl acetate at 0 - 20; for 0.166667 h;Green chemistry;
Steps:
3. General Procedure for the Synthesis of Aryl Mesylates (Table 4)
General procedure: To a solution of hydroxyarene (25.0 mmol) in ethyl acetate (75 mL) at 0 °C was added triethylamine (5.06 g, 50.0 mmol) followed by MsCl (3.72 g, 32.5 mmol). After addition of MsCl, the ice-water bath was removed and the resulting thick slurry was vigorously stirred for 10 min. To the slurry was then added water (50 mL). The two-phase mixture was separated. The organic layer was washed with water (25 mL) and dried over anhydrous MgSO4. Removal of solvent under reduced pressure gave the corresponding pure aryl mesylate.
References:
Lei, Xiangyang;Jalla, Anusha;Abou Shama, Mhd A.;Stafford, Jamie M.;Cao, Billy [Synthesis,2015,vol. 47,# 17,art. no. SS-2015-M0308-PSP,p. 2578 - 2585] Location in patent:supporting information