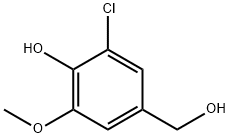
methyl 3-chloro-4-hydroxy-5-methoxybenzoate synthesis
- Product Name:methyl 3-chloro-4-hydroxy-5-methoxybenzoate
- CAS Number:1205-50-1
- Molecular formula:C9H9ClO4
- Molecular Weight:216.62
Yield:1205-50-1 81%
Reaction Conditions:
with potassium carbonate at 55; under 750.075 Torr; for 24 h;
Steps:
S6. Procedure for the synthesis of esters
General procedure: A magnetic stir bar and the alcohol substrate were transferred to 20 mL glass tube and then 2 mL of MeOH oralcohol was added. Then, 35 mg catalyst and 10 mol% of K2CO3 were added. The glass tube containingreaction mixture was fitted with septum and connected to a balloon containing one bar air. Then the glass tubewas placed into a preheated aluminum block at 60°C. Temperature inside the reaction tube was measured tobe 55 oC and this temperature has been taken as the reaction temperature. After completion of the reaction,the glass tube was cooled down to room temperature. Af terwards, the catalyst was f iltered-off and washedwith methanol. The solvent from the filtrate containing the reaction products was removed in vacuum and thecorresponding ester was purified by column chromatography. Products were analyzed by GC-MS and NMRspectroscopy analysis. In the case of yields determined the by GC, 100 μL n-hexadecane was added to thereaction vial containing the products and diluted with ethyl acetate. Then catalyst was f iltered through a plugof silica and the filtrate containing product was analyzed by GC.
References:
Bartling, Stephan;Beller, Matthias;Chandrashekhar, Vishwas G.;Jagadeesh, Rajenahally V.;Rabeah, Jabor;Rockstroh, Nils;Senthamarai, Thirusangumurugan [Chem,2022,vol. 8,# 2,p. 508 - 531] Location in patent:supporting information

62936-23-6
86 suppliers
$54.20/5g

1205-50-1
4 suppliers
inquiry