
methyl 4-(indol-3-yl)butyrate synthesis
- Product Name:methyl 4-(indol-3-yl)butyrate
- CAS Number:15591-70-5
- Molecular formula:C13H15NO2
- Molecular Weight:217.26
67-56-1
733 suppliers
$9.00/25ml
133-32-4
637 suppliers
$10.00/5g

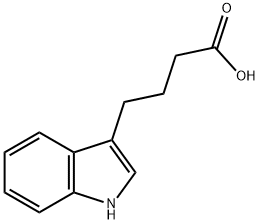
15591-70-5
18 suppliers
$105.00/50mg
Yield:15591-70-5 98%
Reaction Conditions:
with sulfuric acid at 20; for 5 h;
Steps:
53.a
) Methyl 4-(1 H-indol-3-yl)butanoateH2SO4 (3.5 ml_, 64.92 mmol) was added to a solution of indole-3-butyhc acid (4.0 g, 19.68 mmol) in MeOH (100 ml_). The reaction mixture was stirred at r.t. for 5 h, and poured into H2O (150 ml_). It was extracted with CH2CI2 (2x100 ml_). The organic layer was dried over Na2SO4 (anhydrous), filtered and concentrated, to give 4.22 g of methyl 4-(1 H-indol-3-yl)butanoate (white solid, yield: 98%). The compound was submitted to next step without further purification.
References:
WO2009/80722,2009,A2 Location in patent:Page/Page column 79-80

818-57-5
41 suppliers
$123.00/1g
201230-82-2
1 suppliers
inquiry

100-63-0
356 suppliers
$10.00/1g

15591-70-5
18 suppliers
$105.00/50mg

133-32-4
637 suppliers
$10.00/5g

66395-18-4
6 suppliers
inquiry

15591-70-5
18 suppliers
$105.00/50mg

133-32-4
637 suppliers
$10.00/5g
74-88-4
341 suppliers
$15.00/10g

15591-70-5
18 suppliers
$105.00/50mg

133-32-4
637 suppliers
$10.00/5g

15591-70-5
18 suppliers
$105.00/50mg