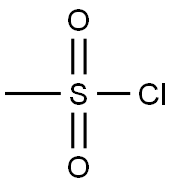METHYLSULFONYLACETONE synthesis
- Product Name:METHYLSULFONYLACETONE
- CAS Number:5000-46-4
- Molecular formula:C4H8O3S
- Molecular Weight:136.17
Yield:-
Reaction Conditions:
Stage #1:methanesulfonyl chloride with sodium hydrogencarbonate;sodium sulfite in water;acetonitrile at 20;
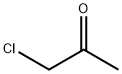
Stage #2:chloroacetone with potassium iodide in water;acetonitrile at 20;
Steps:
α-Diazo-β-ketosulfones 1; General Procedure 1 (GP1)
General procedure: To a stirred solution of Na2SO3 (1.51 g, 12 mmol) and NaHCO3 (2.02 g, 2.4 mmol) in H2O (20 mL) was added a solution of the corresponding sulfonyl chloride (10 mmol) in MeCN (5-10 mL) and the mixture was stirred at r.t. for 12-16 h. To the resulting solution were added the corresponding α-haloketone (11 mmol) and KI (83 mg, 0.5 mmol) and the mixture was stirred for 16-20 h. ‘SAFE’ cocktail [prepared by the addition of 3-(chlorosulfonyl)benzoic acid (2.76 g, 12.5 mmol) to a solution of NaN3 (1.0 g, 15 mmol) and K2CO3 (2.1 g, 15.0 mmol) in H2O (20 mL) and stirring for 10 min] was added to the reaction mixture and stirring was continued for 6 h. If a solid precipitate was formed, the mixture was cooled to 5 °C, the product was filtered, washed with H2O (30 mL) and cold H2O/EtOH mixture (1:1, 25 mL), and dried in air. Oily precipitates were extracted with DCM (3 × 25 mL), the combined organic phases were dried (CaCl2), and evaporated to dryness. Additional purification was used, if needed, by flash column chromatography on silica gel or by recrystallization (as indicated).
References:
Dar'In, Dmitry;Kantin, Grigory;Bakulina, Olga;Krasavin, Mikhail [Synthesis (Germany),2020,vol. 52,# 15,p. 2259 - 2266]

14109-72-9
89 suppliers
$58.50/5g

5000-46-4
69 suppliers
$11.00/1g
20277-69-4
262 suppliers
$10.00/1g

598-31-2
110 suppliers
$60.00/50mg

5000-46-4
69 suppliers
$11.00/1g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
7732-18-5
458 suppliers
$12.69/100ml

5000-46-4
69 suppliers
$11.00/1g
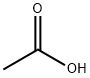
64-19-7
1530 suppliers
$10.00/25ML