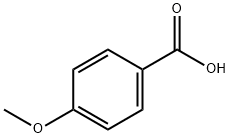
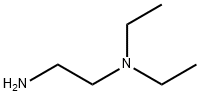
N-[2-(Diethylamino)ethyl]-4-methoxybenzamide, 97% synthesis
- Product Name:N-[2-(Diethylamino)ethyl]-4-methoxybenzamide, 97%
- CAS Number:32276-18-9
- Molecular formula:C14H22N2O2
- Molecular Weight:250.3367
Yield:32276-18-9 88%
Reaction Conditions:
with silica in m-xylene; for 24 h;Dean-Stark;Reflux;Green chemistry;
Steps:
Direct Amide Synthesis from Carboxylic Acids and Amines Catalyzedby Mesoporous Silica SBA-15; General Procedure
General procedure: The amidation was carried out in a 50 mL single-necked round-bottomedflask equipped with a Teflon-coated magnetic stirring bar anda Dean-Stark apparatus. Unless otherwise noted, all reactions wereperformed by using the carboxylic acid 1 (3.0 mmol) and amine 2 (3.0mmol) in anhydrous toluene (15 mL) in the presence of catalyst (0.5g) under reflux conditions for the prescribed reaction period (Tables1-4). After cooling to r.t., the suspended catalyst was removed by filtrationand rinsed with CHCl3 (10 mL). The collected small amount ofan aliquot filtrate was then analyzed by gas chromatography to determinethe yield of amide (Shimadzu 18A, column: Ultra ALLOY+-65) bycomparing with an authentic sample. All reactions produce the correspondingamide without any by-product and residual substrate. Theremaining filtrate was purified by column chromatography to givethe pure product. The filtered catalyst was subjected to calcinationunder air flow (10 mL/min) at 550 °C for 5 h to evacuate the occludedorganic residue.
References:
Tamura, Mizuki;Murase, Daisuke;Komura, Kenichi [Synthesis,2015,vol. 47,# 6,art. no. SS-2014-F0617-OP,p. 769 - 776] Location in patent:supporting information
![Carbamic acid, N-[2-[(4-methoxybenzoyl)amino]ethyl]-, 1,1-dimethylethyl ester](/CAS/20200611/GIF/1227301-63-4.gif)
1227301-63-4
0 suppliers
inquiry
![N-[2-(Diethylamino)ethyl]-4-methoxybenzamide, 97%](/CAS/20180528/GIF/32276-18-9.gif)
32276-18-9
1 suppliers
inquiry
100-07-2
389 suppliers
$35.00/25g
![N-[2-(Diethylamino)ethyl]-4-methoxybenzamide, 97%](/CAS/20180528/GIF/32276-18-9.gif)
32276-18-9
1 suppliers
inquiry