
NEOPENTYLBENZENE synthesis
- Product Name:NEOPENTYLBENZENE
- CAS Number:1007-26-7
- Molecular formula:C11H16
- Molecular Weight:148.24
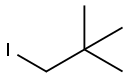
15501-33-4
55 suppliers
$15.00/1g

100-58-3
183 suppliers
$18.00/10ml

1007-26-7
83 suppliers
$36.79/5g
Yield: 15 %Chromat.
Reaction Conditions:
Stage #1:phenylmagnesium bromide with zinc(II) chloride in tetrahydrofuran at -20; for 0.25 h;Inert atmosphere;
Stage #2: with methylmagnesium chloride in tetrahydrofuran at -20; for 0.25 h;Inert atmosphere;
Stage #3:neopentyl iodide with triphenylphosphine;nickel dichloride in tetrahydrofuran at 0 - 20; for 1 h;Inert atmosphere;regioselective reaction;
Steps:
Typical procedure for the coupling of methylarylzincs with primary alkyl iodides:
General procedure: All reactions were carried out in oven-dried glassware under a positive pressure of nitrogen using standard syringe-septum cap techniques.28 For the preparation of methylarylzinc reagents (aryl: C6H5, MeC6H4, MeOC6H4), arylzinc chlorides were reacted with methylmagnesium chloride. Arylzinc chloride was prepared by addition of arylmagnesium bromide (2 mmol) to ZnCl2 (2 mmol) in THF (4 ml) at -20 °C and stirring at that temperature for 15 min. To freshly prepared arylzinc chloride (2 mmol), methylmagnesium chloride (2 mmol) was added and the mixture was stirred at -20 °C for another 15 min. For the preparation of methylarylzinc reagents (aryl: 4-MeCOC6H4, 3-MeCOC6H4), arylmagnesium bromides were reacted with methylzinc chloride.26 Arylmagnesium bromide (2 mmol) was prepared by addition of isopropylmagnesium bromide (2.1 mmol) to a solution of aryl iodide (2 mmol) in THF (4 ml) at -15 °C and stirring at that temperature for 1 hour to complete the Br/Mg exchange.29 Arylmagnesium bromide (2 mmol) solution was added to methylzinc chloride (2 mmol), freshly prepared by addition of methylmagnesium bromide (2 mmol) to ZnCl2 (2 mmol) in THF (4 ml) at -20 °C and the mixture was stirred at that temperature for 15 min. For the alkyl coupling reaction of methylarylzincs, a mixture of NiCl2 (10 mol %, 0.013 g), PPh3 (10 mol %, 0.0263) and primary alkyl iodide in THF (2 ml) was cooled to 0 °C and freshly prepared methylarylzinc (2 mmol) was added slowly. The reaction mixture was stirred at room temperature for 1 h and then hydrolyzed by addition of 1 M HCl and subsequently extracted with Et2O. The combined ethereal solutions were washed with aq NaHCO3 solution, dried and aliquots were analyzed by GC.
References:
Pekel, Özgen Ömür;Erdik, Ender [Tetrahedron Letters,2011,vol. 52,# 52,p. 7087 - 7090] Location in patent:experimental part

938-16-9
73 suppliers
$14.00/100mg

1007-26-7
83 suppliers
$36.79/5g

558-17-8
69 suppliers
$60.00/500mg
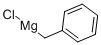
6921-34-2
218 suppliers
$53.20/100ml

1007-26-7
83 suppliers
$36.79/5g
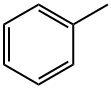
108-88-3
7 suppliers
$21.80/5 mL
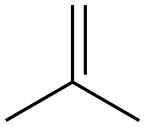
115-11-7
228 suppliers
$45.00/25 g

1007-26-7
83 suppliers
$36.79/5g

