
(R)-Styrene oxide synthesis
- Product Name:(R)-Styrene oxide
- CAS Number:20780-53-4
- Molecular formula:C8H8O
- Molecular Weight:120.15

70-11-1
288 suppliers
$10.00/25g

20780-53-4
265 suppliers
$18.00/1g
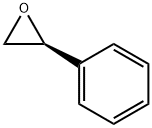
20780-54-5
195 suppliers
$17.00/1g
Yield: 81 % ee
Reaction Conditions:
with tris(triphenylphosphine)ruthenium(II) chloride;(3aR,5R,7R,7aS)-2-(2-(diphenylphosphino)phenyl)-6,6-dimethyl-3a,4,5,6,7,7a-hexahydro-5,7-methanobenzo[d]oxazole;isopropyl alcohol;sodium hydroxide for 0.5 h;Inert atmosphere;Reflux;Overall yield = 68 %; enantioselective reaction;
Steps:
4.10. Transfer hydrogenation of acetophenone. General procedure
General procedure: In a two-neck flask (25 mL), under an inert atmosphere, wereplaced a catalyst solution (0.05 M in isopropanol, 100 lL, 5 lmol),a degassed 0.125 M solution of sodium hydroxide or potassium tert-butoxide in isopropanol (1 mL, 0.125 mmol), and degassed isopropanol(4 mL). After stirring for 15 min. at room temperature acetophenone (120 mg, 1 mmol) was added and the reaction mixture was refluxed for 30 min. Isopropanol was removed on rotary evaporator and 1-phenylethanol was isolated by flash column chromatography on silica gel (eluent: n-hexane:ethyl acetate80:20).
References:
Kmieciak, Anna;Krzemiński, Marek P. [Tetrahedron Asymmetry,2017,vol. 28,# 3,p. 467 - 472]

292638-84-7
0 suppliers
inquiry

20780-53-4
265 suppliers
$18.00/1g

343239-88-3
0 suppliers
inquiry

20780-53-4
265 suppliers
$18.00/1g

292638-84-7
0 suppliers
inquiry

20780-53-4
265 suppliers
$18.00/1g

20780-54-5
195 suppliers
$17.00/1g
100-52-7
947 suppliers
$17.30/2g

73908-23-3
20 suppliers
inquiry

20780-53-4
265 suppliers
$18.00/1g