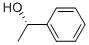
(S)-(-)-1-PHENYLETHANOL synthesis
- Product Name:(S)-(-)-1-PHENYLETHANOL
- CAS Number:1445-91-6
- Molecular formula:C8H10O
- Molecular Weight:122.16
Yield:1445-91-6 100%
Reaction Conditions:
with RuBr2[(S,S)-2,4-bis(diphenylphosphino)pentane](2-aminomethyl-3,5-dimethylpyridine);potassium tert-butylate;hydrogen in ethanol at 40; under 7600.51 Torr; for 19 h;Inert atmosphere;Autoclave;Reagent/catalyst;
Steps:
1 The Hydrogenating Reaction of Acetophenone
In an autoclave, 1.32 mg of RuBr2[(S,S)-xylskewphos] (3,5-Me2pica) (1.29×10-3 mmol, S/C=10000) and 5.79 mg of potassium tert-butoxide (5.16×10-2 mmol) are placed, and replaced with argon gas. Under argon gas flow, 1.5 mL of acetophenone (12.9 mmol) and 2.9 mL of ethanol was added while measuring by a syringe, pressurized with hydrogen to 10 atm, stirred at 40° C. for 19 hours, then the reduction of the hydrogen pressure was confirmed and phenylethanol was obtained at 100% yield. The optical purity was 88.0% ee as measured by GC (CP-Chirasil-DEX CB (0.25 mml. D×25 m, DF=0.25 μm, from VARIAN), constant at 110° C., pressure: 102.0 kPa, column flow: 1.18 mL/min, vaporizing chamber temperature: 250° C., detector temperature: 275° C., the retention time of each enantiomer was: (R): 11.7 min, (S): 12.4 min), and (S) isomer has predominantly been generated.
References:
KATAYAMA, TAKEAKI;TSUTSUMI, KUNIHIKO;MURATA, KUNIHIKO;OHKUMA, TAKESHI;ARAI, NORIYOSHI US2015/31920, 2015, A1 Location in patent:Paragraph 0159; 0160
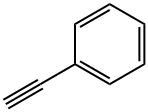
536-74-3
293 suppliers
$10.00/1g
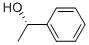
1445-91-6
285 suppliers
$6.00/1g

292638-84-7
0 suppliers
inquiry
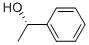
1445-91-6
285 suppliers
$6.00/1g
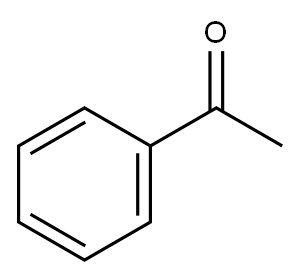
98-86-2
738 suppliers
$9.00/5g
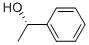
1445-91-6
285 suppliers
$6.00/1g
585-74-0
180 suppliers
$10.00/5g
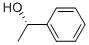
1445-91-6
285 suppliers
$6.00/1g
