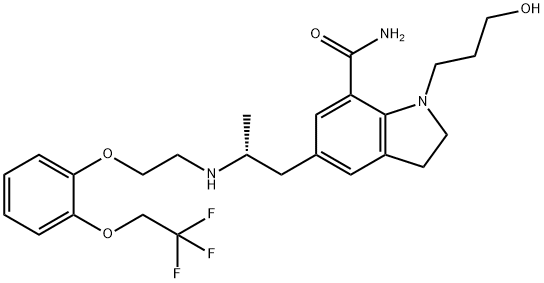
Silodosin synthesis
- Product Name:Silodosin
- CAS Number:160970-54-7
- Molecular formula:C25H32F3N3O4
- Molecular Weight:495.53

Yamaguchi, Toshiaki; Tsuchiya, Ikuo; Kikuchi, Ken; Yanagi, Takashi. Process for preparation of silodosin. Assignee Kissei Pharmaceutical Co., Ltd., Japan. WO 2006046499. (2006).
![1H-Indole-7-carbonitrile, 2,3-dihydro-1-(3-hydroxypropyl)-5-[(2R)-2-[[2-[2-(2,2,2-trifluoroethoxy)phenoxy]ethyl]aMino]propyl]-](/CAS/GIF/885340-13-6.gif)
885340-13-6
40 suppliers
inquiry

160970-54-7
391 suppliers
$6.00/5mg
Yield:160970-54-7 96.24%
Reaction Conditions:
Stage #1: 1-(3-hydroxypropyl)-5-[(2R)-2-({2-[2-(2,2,2-trifluoroethoxy)phenoxy]ethyl}amino)propyl]-2,3-dihydro-1H-indole-7-carbonitrilewith potassium carbonate in water; pH=9;
Stage #2: with cesium hydroxide;dihydrogen peroxide in dimethyl sulfoxide at 20; for 2 h;
Steps:
18 Example 18 1- (3-hydroxypropyl) -5-[(2R) -2- [2- [2- (2,2,2-trifluoroethoxy) phenoxy] ethyl] amino] propyl] indoline-7-carboxamideProduction of (compound of formula 1, silodosin)
Manufactured in Example 171- (3-hydroxypropyl) -5-[(2R) -2- [2- [2- (2,2,2-trifluoroethoxy) phenoxy] ethyl] aminopropyl] indoline-7-carbonitrile(Compound of Chemical Formula 2a) Oxalate (25 g, 44 mmol)Is suspended in purified water (100 mL),The pH was adjusted to 9 or more by adding 1 N K2CO3 solution.The reaction mixture was extracted with dichloromethane (100 mL × 2).The combined organic layers were dried over MgSO 4 and filtered.The filtrate is concentrated under reduced pressure,Vacuum dried.The resulting product is dissolved in 60 ml of dimethyl sulfoxide and cooled to 20 ° C. or less.30% hydrogen peroxide (15.2 mL, 2 equivalents) was added slowly.Add cesium hydroxide (12.7 g, 1 equivalent) at the same temperature,The temperature was gradually raised to room temperature and vigorously stirred for 2 hours.Add 5% aqueous sodium sulfite solution (120 mL),Extracted with dichloromethane (100 mL × 2).Combine the organic layers and wash thoroughly with water and brine,After drying over anhydrous sodium sulfate, it was filtered. The filtrate is concentrated under reduced pressure, dried under vacuum, and the residue is recrystallized with ethyl acetate (120 mL).Vacuum dry,21 g of the title compound were obtained.(Yield: 96.24%,HPLC purity: 99.95%)
References:
JP2019/31488,2019,A Location in patent:Paragraph 0054
![tert-butyl (2R)-1-[1-(3-benzyloxypropyl)-7-carbamoyl-indolin-5--yl]propan-2-yl2-[2-(2,2,2-trifluoroethoxy)phenoxy]ethylcarbamate](/CAS/20180629/GIF/175870-34-5.gif)
175870-34-5
0 suppliers
inquiry

160970-54-7
391 suppliers
$6.00/5mg
![1-[3-(Benzoyloxy)propyl]-2,3-dihydro-5-[(2R)-2-[[2-[2-(2,2,2-trifluoroethoxy)phenoxy]ethyl]amino]propyl]-1H-indole-7-carbonitrile](/CAS/GIF/885340-11-4.gif)
885340-11-4
23 suppliers
inquiry

160970-54-7
391 suppliers
$6.00/5mg
![2,3-DIHYDRO-1-[3-(PHENYLMETHOXY)PROPYL]-5-[(2R)-2-[[2-[2[(2,2,2-TRIFLUOROETHOXY)PHENOXY]ETHYL]
AMINO]PROPYL]-1H-INDOLE-7-CARBONITRILE](/CAS/20210305/GIF/459868-77-0.gif)
459868-77-0
1 suppliers
inquiry

160970-54-7
391 suppliers
$6.00/5mg
![5-[(2R)-2-Aminopropyl]-1-[3-(benzoyloxy)propyl]-2,3-dihydro-7-carbonitrile-1H-indole](/CAS/20180808/GIF/239463-72-0.gif)
239463-72-0
18 suppliers
inquiry
![[2-(2,2,2-trifluoroethoxy)phenoxy]acetaldehyde](/CAS/GIF/1269801-73-1.gif)
1269801-73-1
15 suppliers
inquiry

160970-54-7
391 suppliers
$6.00/5mg








