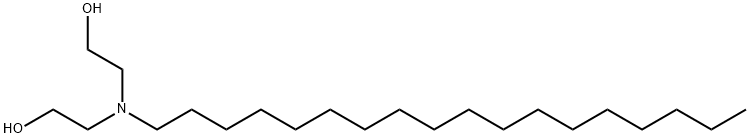
STEARYLDIETHANOLAMINE synthesis
- Product Name:STEARYLDIETHANOLAMINE
- CAS Number:10213-78-2
- Molecular formula:C22H47NO2
- Molecular Weight:357.61
111-42-2
817 suppliers
$10.00/20mg

112-89-0
343 suppliers
$5.00/25g

10213-78-2
141 suppliers
$5.00/1g
Yield:10213-78-2 100%
Reaction Conditions:
with potassium hydrogencarbonate;potassium iodide in acetonitrile for 3 h;Heating / reflux;
Steps:
4.4-1
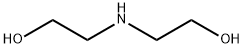
In a 500 ml Erlenmeyer flask, 10 g (95 mmol) of diethanolamine, 37.96 g (0.114 mol) of octadecane bromide, 39.33 g (0.285 mol) of potassium bicarbonate and 0.5 g of potassium iodide in 200 ml of acetonitrile were mixed. The reaction mixture was stirred and heated under reflux for 3 hours. At the end of the reaction, the solvent was evaporated and the residue was taken up in dichloromethane. The organic phase was washed two times with water, dried over MgSO4, filtered, and concentrated under vacuum. The residue was crystallized from acetone, to obtain 33 g of white crystal. Yield: quantitative. Melting point: 49° C. Rf: 0.20 (CH2Cl2/MeOH, 90:10 v/v). IR (KBr): 3310 (O-H) cm-1 1H NMR (200 MHz, CDCl3, HMDS) δ ppm: 3.53 (t, 4H, J=5.43 Hz, CH2-O), 3.27 (s1, 2H, OH), 2.57 (t, 4H, J=5.43 Hz, N-CH2-C-O), 2.44 (t, 2H, J=7.06 Hz, CH2-N), 1.34 (m, 2H, CH2-C-N), 1.18 (s1, 30H, CH2), 0.80 (t, 3H, J=5.85 Hz, CH3)
References:
Yang Ji Chemical Company Ltd. US2005/75345, 2005, A1 Location in patent:Page/Page column 7

3386-33-2
261 suppliers
$13.00/25g

111-42-2
817 suppliers
$10.00/20mg

10213-78-2
141 suppliers
$5.00/1g

75-21-8
213 suppliers
$33.29/crm44609

124-30-1
393 suppliers
$8.00/25g

10213-78-2
141 suppliers
$5.00/1g