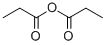
Tripropionin synthesis
- Product Name:Tripropionin
- CAS Number:139-45-7
- Molecular formula:C12H20O6
- Molecular Weight:260.28

802294-64-0
1 suppliers
inquiry
56-81-5
1624 suppliers
$5.00/25g

139-45-7
95 suppliers
$19.00/25g
624-47-5
2 suppliers
inquiry

18373-34-7
0 suppliers
inquiry
18373-31-4
19 suppliers
inquiry

18462-47-0
0 suppliers
inquiry
Yield:139-45-7 94.3%
Reaction Conditions:
scandium tris(trifluoromethanesulfonate) in toluene at 129;
Steps:
2
Example 2Synthesis of Glycerol Esters of PropionateGlycerol was esterified with propionic acid by mixing 31.5 ml (0.27 mole) of glycerol and 0.6 g (0.0012 mole) of Sc(OCF3SO2-)3 in a 500-ml three-neck, round-bottom flask with a stir bar. Then 300 ml of propionic acid and 50 ml of toluene were added. The flask was attached to a thermocouple, a solvent trap with a reflex condenser, and a heating mantle. The mixture was stirred and heated to 129° C. The reaction was followed by measuring the volume of water azeotropically removed from the reaction. In a typical reaction of this scale, approximately 14.7 g of reaction water was removed. The reaction was also followed by GC. For this, the reaction was cooled, and 50 μl of the reaction solution was combined with 1 ml of BSTFA. The mixture was allowed to stand at room temperature for 2 hours before injection into the GC.Upon completion of the reaction, the reaction mixture was cooled, transferred to a single neck flask, and the toluene was removed by rotary evaporation. The residue was transferred to a vacuum distillation system and excess propionic acid was removed by vacuum distillation. The crude product was transferred to a suitable flask and distilled under high vacuum. Analysis of the reaction products by GC and GC-MS revealed that the most abundant species was the triester of propionoate, with a yield of 94.3% at 97.7% purity. A small amount of the diester was present (0.7%), and only a trace of the monoester was present.
References:
US2007/292485,2007,A1 Location in patent:Page/Page column 15