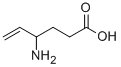
VIGABATRIN synthesis
- Product Name:VIGABATRIN
- CAS Number:60643-86-9
- Molecular formula:C6H11NO2
- Molecular Weight:129.16

Deleris, G.; Dunogues, J.; Gadras, A. Direct regiospecific allylic amination via silicon induced pericyclic reactions. A novel synthesis of gamma vinyl GABA. Tetrahedron. Volume 44. Issue 13. Pages 4243-58. 1988.
7529-16-0
93 suppliers
$53.00/1g

60643-86-9
167 suppliers
$75.00/10 mg
Yield:60643-86-9 60%
Reaction Conditions:
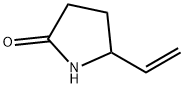
Stage #1:5(S)-Vinyl-2-Pyrrolidinone with water;potassium hydroxide in isopropyl alcohol at 20 - 60; for 16 h;Inert atmosphere;
Stage #2: with acetic acid in water;isopropyl alcohol
Steps:
c
Step c) 5-vinyl-2-pyrrolidone (III) hydrolysis to 4-amino-5-hexenoic acid (I): 5-vinyl-2-pyrrolidone (III) can be hydrolyzed to 4-amino-5-hexenoic acid of formula (I), according to one of the two procedures described in or . Under argon, 1 g of 5-vinyl-2-pyrrolidone (6 mmol) is dissolved in a mixture of isopropanol (10 ml) and deionized water (1 ml). To this solution, 0.9 g of potassium hydroxide is added at room temperature and the mixture is then heated at 60°C for 16 h. After cooling, the reaction is quenched with 1 ml of glacial acetic acid and crude 4-amino-5-hexenoic acid precipitates in the reaction mixture. 4-amino-5-hexenoic acid is recovered by filtration and recrystallized in a mixture of water/isopropanol to give 700 mg of white 4-amino-5-hexenoic acid crystals (60 % yield).
References:
Targeon SAS EP2537827, 2012, A1 Location in patent:Page/Page column 9

144085-15-4
0 suppliers
inquiry

60643-86-9
167 suppliers
$75.00/10 mg
![Carbamic acid, [1-(2-cyanoethyl)-2-propenyl]-, phenylmethyl ester (9CI)](/CAS/20210305/GIF/440366-57-4.gif)
440366-57-4
0 suppliers
inquiry

60643-86-9
167 suppliers
$75.00/10 mg

54653-25-7
19 suppliers
$210.00/1g

60643-86-9
167 suppliers
$75.00/10 mg

31696-00-1
8 suppliers
inquiry

60643-86-9
167 suppliers
$75.00/10 mg





