| Identification | Back Directory | [Name]
Ceritinib (LDK378) | [CAS]
1032900-25-6 | [Synonyms]
LDK378
Eritinib
ceritinib
LDK378/LDK-378
LDK378 Ceritinib
Ceritinib, >=98%
Eritinib (LDK378)
Ceritinib (LDK378)
5-Chloro-N2-(2-isopropoxy-5-methyl-4-(piperidin-4-yl)phenyl)-N4-(2-(isopropylsulfonyl)phenyl)p
5-Chloro-N2-[2-isopropoxy-5-Methyl-4-(4-piperidyl)phenyl]-N4-(2-isopropylsulfonylphenyl)pyriMidine-2,4-diaMine
5-chloro-N2-(2-isopropoxy-5-Methyl-4-(piperidin-4-yl)phenyl)-N4-(2-(isopropylsulfonyl)phenyl)pyriMidine-2,4-diaMine
5-Chloro-N4-[2-[(1-Methylethyl)sulfonyl]phenyl]-N2-[5-Methyl-2-(1-Methylethoxy)-4-(4-piperidinyl)phenyl]-2,4-pyriMidinediaMine
2,4-Pyrimidinediamine, 5-chloro-N4-[2-[(1-methylethyl)sulfonyl]phenyl]-N2-[5-methyl-2-(1-methylethoxy)-4-(4-piperidinyl)phenyl]-
5-Chloro-N4-[2-[(1-methylethyl)sulfonyl]phenyl]-N2-[5-methyl-2-(1-methylethoxy)-4-(4-piperidinyl)phenyl]-2,4-pyrimidinediamine LDK 378
LDK 378 5-Chloro-N4-[2-[(1-methylethyl)sulfonyl]phenyl]-N2-[5-methyl-2-(1-methylethoxy)-4-(4-piperidinyl)phenyl]-2,4-pyrimidinediamine | [EINECS(EC#)]
811-457-8 | [Molecular Formula]
C28H36ClN5O3S | [MDL Number]
MFCD26142648 | [MOL File]
1032900-25-6.mol | [Molecular Weight]
558.135 |
| Chemical Properties | Back Directory | [Melting point ]
173-175°C | [Boiling point ]
720.7±70.0 °C(Predicted) | [density ]
1.251±0.06 g/cm3(Predicted) | [storage temp. ]
-20°C Freezer | [solubility ]
Chloroform (Slightly), Methanol (Slightly) | [form ]
Off-white solid. | [pka]
10.16±0.10(Predicted) | [color ]
White to Off-White |
| Hazard Information | Back Directory | [Description]
Ceritinib (previously LDK378l, brand name Zykadia; Novartis Pharmaceuticals) is
an oral small molecule tyrosine kinase inhibitor of ALK [87]. Preclinical
studies suggested that it would inhibit ROS1 as well [88, 89]. | [Uses]
5-Chloro-N2-(2-isopropoxy-5-methyl-4-(piperidin-4-yl)phenyl)-N4-(2-(isopropylsulfonyl)phenyl)pyrimidine-2,4-diamine, is a Anaplastic lymphoma kinase (ALK) inhibitor. | [Definition]
ChEBI: A member of the class of aminopyrimidines that is 2,6-diamino-5-chloropyrimidine in which the amino groups at positions 2 and 6 are respectively carrying 2-methoxy-4-(piperidin-4-yl)-5-methylphenyl and 2-(isopropylsulfonyl)phenyl substituents. Used for the
treatment of ALK-positive metastatic non-small cell lung cancer. | [Indications]
Ceritinib (Zykadia(R), Novartis), approved in 2014, was developed as a second-generation ALK inhibitor for patients with NSCLC who have developed crizotinib resistance. Ceritinib addresses two of the most common ALK mutants that lead to crizotinib resistance, L1196M andG1269A, but is ineffective for G1202R and F1174C variants of ALK. | [General Description]
Class: receptor tyrosine kinase; Treatment: NSCLC;
Elimination half-life = 41 h;
Protein binding = 97% | [Trade name]
Zykadia? | [Clinical Use]
ALK-inhibitor:
Treatment of anaplastic lymphoma kinase (ALK)-
positive advanced non-small cell lung cancer
(NSCLC) previously treated with crizotinib | [Synthesis]
The critical step in the synthesis of ceritinib involves a latestage
Buchwald¨CHartwig coupling of two advanced intermediates,
anilino piperidine 50 and arylsulfonyl chloro-pyrimidine 51. While these conditions utilize microwave-mediated
conditions (as does another Suzuki coupling within the sequence),
which are not commonly employed for process-scale routes, to our
knowledge no other conditions to have been reported to date
which facilitate the construction of ceritinib.
Construction of anilino piperidine 50 commenced with 2-
chloro-4-fluoro-1-methylbenzene (45). Nitration with KNO3/
H2SO4 and subsequent reaction with i-PrOH/Cs2CO3 at elevated
temperatures provided the 5-isopropoxy chloride intermediate
46 in 67% over 2 steps. Suzuki coupling of 46 with 4-pyridine boronic
acid (47) furnished 49 in 73% yield, which was then subjected
to platinum oxide-catalyzed hydrogenation conditions in the presence
of acetic acid and trifluoroacetic acid, affording the corresponding
piperidinyl aniline intermediate. Immediate Bocprotection
of the crude aniline provided the Buchwald¨CHartwig
coupling precursor 50 in 60% over 2 steps. Next, the critical union
of 50 and 51 via Buchwald¨CHartwig coupling furnished the framework
of ceritinib. This was followed by removal of the Boc group
with TFA and subsequent precipitation with 1 M HCl to yield ceritinib
(VII) as the HCl salt in 35% yield from 50.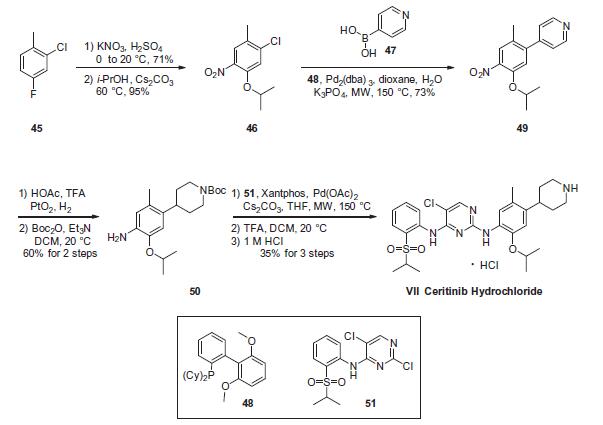
| [target]
ALK | [Drug interactions]
Potentially hazardous interactions with other drugs
Anti-arrhythmics: possibly increased risk of
ventricular arrhythmias with amiodarone,
disopyramide, dronedarone and flecainide.
Antibacterials: possibly increased risk of ventricular
arrhythmias with bedaquiline, clarithromycin,
delamanid, IV erythromycin, moxifloxacin and
telavancin; concentration reduced by rifampicin and
possibly rifabutin - avoid.
Antidepressants: risk of QT prolongation with
citalopram, escitalopram, venlafaxine and tricyclics
that prolong the QT interval - avoid; concentration
possibly reduced by St John’s wort - avoid.
Anti-emetics: possibly increased risk of ventricular
arrhythmias with domperidone and ondansetron.
Antiepileptics: possibly increased concentration
with carbamazepine - avoid; concentration possibly
reduced by fosphenytoin, phenobarbital, phenytoin
and primidone.
Antifungals: concentration increased by ketoconazole
and possibly itraconazole, posaconazole and
voriconazole - avoid or reduce ceritinib dose
Antihistamines: avoid with hydralazine due to risk of
QT prolongation.
Antimalarials: possibly increased risk of ventricular
arrhythmias with artemether and lumefantrine,
piperaquine with artenimol, chloroquine and quinine
- avoid.
Antipsychotics: possibly increased risk of ventricular
arrhythmias with droperidol and haloperidol;
avoid with other antipsychotics that prolong the
QT interval; increased risk of agranulocytosis with
clozapine - avoid.
Antivirals: concentration possibly increased by
atazanavir, fosamprenavir, lopinavir, ritonavir,
saquinavir and tipranavir - avoid or reduce dose; risk
of QT prolongation with dasatinib - avoid.
Apomorphine: risk of QT prolongation - avoid.
Beta-blockers: possibly increased risk of ventricular
arrhythmias with sotalol.
Ciclosporin: may increase ciclosporin concentration
- avoid.
Cobicistat: concentration of ceritinib increased -
avoid or adjust ceritinib dose.
Cytotoxics: risk of QT prolongation with arsenic
trioxide, bosutinib, cabozantib, crizotinib, eribulin,
lapatinib, nilotinib, osimertinib, panobinostat,
pazopanib, sorafenib, sunitinib, vandetanib,
vemurafenib, vinflunine - avoid; concentration possibly increased by idelalisib - avoid or adjust
ceritinib dose
Enzalutamide: increases ceritinib concentration -
avoid.
Methadone: possibly increased risk of ventricular
arrhythmias.
Pasireotide: possibly increased risk of ventricular
arrhythmias - avoid.
Ranolazine: possibly increased risk of ventricular
arrhythmias - avoid.
Sirolimus: avoid concomitant use.
Tacrolimus: avoid concomitant use.
Tetrabenazine: possibly increased risk of ventricular
arrhythmias - avoid.
Tizanidine: possibly increased risk of ventricular
arrhythmias - avoid.
Warfarin - avoid concomitant use. | [Metabolism]
In vitro studies demonstrated that CYP3A was the
major enzyme involved in the metabolic clearance of
ceritinib. The main route of excretion of ceritinib and
its metabolites is via the faeces. Recovery of unchanged
ceritinib in the faeces accounts for a mean of 68% of a
dose. | [storage]
Store at +4°C | [References]
[1]chen j, jiang c, wang s. ldk378: a promising anaplastic lymphoma kinase (alk) inhibitor. j med chem. 2013 jul 25;56(14):5673-4. doi: 10.1021/jm401005u. epub 2013 jul 9.
[2]marsilje th, pei w, chen b, lu w, uno t, jin y, jiang t, kim s, li n, warmuth m, sarkisova y, sun f, steffy a, pferdekamper ac, li ag, joseph sb, kim y, liu b, tuntland t, cui x, gray ns, steensma r, wan y, jiang j, chopiuk g, li j, gordon wp, richmond w, johnson k, chang j, groessl t, he yq, phimister a, aycinena a, lee cc, bursulaya b, karanewsky ds, seidel hm, harris jl, michellys py. synthesis, structure-activity relationships, and in vivo efficacy of the novel potent and selective anaplastic lymphoma kinase (alk) inhibitor 5-chloro-n2-(2-isopropoxy-5-methyl-4-(piperidin-4-yl)phenyl)-n4-(2-(isopropylsulfonyl)phenyl)pyrimidine-2,4-diamine (ldk378) currently in phase 1 and phase 2 |
| Questions And Answer | Back Directory | [Indications and Usage]
Ceritinib is a new ALK gene inhibitor (ALKi) developed by Novartis Pharmaceuticals; its commercial name is Zykadia, and its previous code was LDK378. It was approved by the FDA for sale on April 29, 2014, and it is used to treat anaplastic lymphoma kinase (ALK) positive transfer of crizotinib (CRZ) progress or intolerance of non-small cell lung cancer (NSCLC).
| [Mechanisms of Action]
Crizotinib resistance is a major issue for patients undergoing treatment for ALK gene rearrangement positive non-small cell lung cancer. Ceritinib is a kind of ALK tyrosine kinase inhibitor. Ceritinib does not target the MET proto-oncogene, but instead inhibits the insulin-like growth factor 1 receptor and blocks proteins from promoting cancer cell development, thus inhibiting the expression of EML4-ALK and NPM-ALK fusion protein cells. It is used to treat ALK rearrangement positive NSCLC patients who have previously used Crizotinib and can overcome Crizotinib resistance. Compared to Crizotinib, Ceritinib does not inhibit MET kinase activity, but inhibits IGF-1 receptors. Whether in terms of enzyme reaction, cell analysis or Crizotinib resistant animal models, research results all show that Ceritinib is more effective than Crizotinib. Additionally, regardless of any ALK resistance mutation, Ceritinib is still highly effective. In clinical models, Ceritinib’s ALK inhibition has 20 times the tumor-fighting effect of Crizotinib. Ceritinib also has the same effect on Crizotinib resistant central nervous system lesion NSCLC. Clinical trials show that Ceritinib can effectively inhibit ALK targets, potentially affecting an unknown kinase related to drug resistance, thus overcoming Crizotinib resistance.
| [Binding Mode]
Ceritinib is a type I inhibitor of ALK, and it binds
similarly to crizotinib with the DFG-Asp in
conformation (Fig. 3). The 2,4-diaminopyrimidine
core forms two hydrogen bonds with Met1199 of the
ALK hinge (Fig. 4). One of the sulfonyl oxygens
forms a 6-membered intramolecular hydrogen bond
with the adjacent amine, and this oxygen also
interacts with the side chain amine of Lys1150 via a
water molecule. The terminal 2-isopropoxy-3-
(piperidin-4-yl) phenyl group resides between the
solvent and the ATP-binding pocket.
  | [Patents]
American patent numbers: US7153964,US7893074,US7964592,US8039474,US8039479,US8377921,US8703787.
Patent expiration dates:
February 26, 2021 (US7153964)
April 25, 2026 (US7893074)
January 13, 2027 (US7964592)
June 29, 2030 (US8039474, US8039479)
November 20, 2027 (US8377921)
April 29, 2032 (US8703787)
Patents belong to Novartis
| [Chemical Synthesis]
Ceritinib can be synthesized using a highly convergent synthetic route that consist two sequential amination reactions on 2,4,5-trichloropyrimidine itself (Scheme 6.1). In the first amination step, 2-(isopropylsulfonyl)aniline can be isolated in three steps from fluoronitrobenzene, and in the second step, 2-isopropoxy-5-methyl-4-(piperidin-4-yl)aniline is easily isolated in four steps from 2-chloro-4-fluorotoluene. Overall yields are >28% from 2-fluoronitrobenzene and >22% from 2-chloro-4-fluorotoluene.
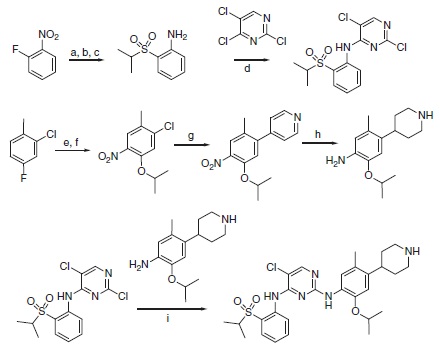
Scheme 6.1 Synthesis of LDK378 (1, ceritinib). Reagents and conditions: (a) propane-2-thiol, K2CO3, DMF, 45°C ON. (b) NaBO3, AcOH, 60°C. (c) H2/Pd/C, EtOAc/MeOH (10/1). (d) NaH, DMF/DMSO, 0–20 °C. (e) KNO3, H2SO4, 0–20°C. (f ) IPA, Cs2CO3, 60°C, 24 h. (g) 4-Pyridineboronic acid, 1-BuOH (Pd2(dba)3, 2-dicyclohexylphosphine-2′-6′-dimethoxy biphenyl, MW, 150 °C. (h) AcOH/TFA; PtO2, H2, RT, 3 h. (i) Anh. HCl-dioxane, 0.1M anh. 2-methoxy ethanol, 135 °C, 2 h.
|
|
|