| Identification | Back Directory | [Name]
hexahydro-3,6-methanophthalic anhydride | [CAS]
6004-79-1 | [Synonyms]
Einecs 227-852-8
hexahydro-3,6-methanophthalic anhydride
4-Oxatricyclo[5.2.1.02,6]decane-3,5-dione
Hexahydro-4,7-Methanoisobenzofuran-1,3-dione
4,7-Methanoisobenzofuran-1,3-dione, hexahydro-
Bicyclo[2.2.1]heptane-2,3-dicarboxylic anhydride
3a,4,5,6,7,7a-Hexahydro-4,7-methanoisobenzofuran-1,3-dione | [EINECS(EC#)]
227-852-8 | [Molecular Formula]
C9H10O3 | [MDL Number]
MFCD00143693 | [MOL File]
6004-79-1.mol | [Molecular Weight]
166.17 |
| Chemical Properties | Back Directory | [Boiling point ]
325.2±11.0 °C(Predicted) | [density ]
1.345 | [InChI]
InChI=1S/C9H10O3/c10-8-6-4-1-2-5(3-4)7(6)9(11)12-8/h4-7H,1-3H2 | [InChIKey]
LQOPXMZSGSTGMF-UHFFFAOYSA-N | [SMILES]
C1(=O)C2C(C3CC2CC3)C(=O)O1 |
| Hazard Information | Back Directory | [Synthesis]
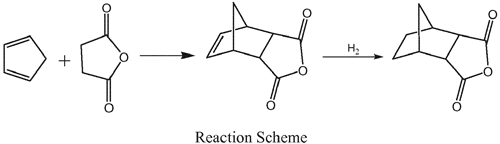
The production method uses maleic anhydride and mixed C5s as raw materials, generates Nadic anhydride by absorbing cyclopentadiene with maleic anhydride directly at a temperature of 0-5°C, and crystallizes to obtain a pure product; and a Cu-Ni/ZnO-ZrO2 or Co-Ni/ZnO-ZrO2 catalyst is added to the generated Nadic anhydride, carrying out a catalytic hydrogenation reaction in a high-pressure autoclave for 4-6 hours at a temperature of 100-140°C and under a hydrogen pressure of 1-4 MPa, the catalyst is filtered after cooling, and reduced pressure distilling to obtain the hexahydro-3,6-methanophthalic anhydride.[1]
 | [References]
[1] Endo-methylene hexahydrophthalic anhydride and production method thereof. Patent EP2495241A1. |
|
|