| Identification | Back Directory | [Name]
Prednisone Impurity 13 | [CAS]
91160-89-3 | [Synonyms]
BNKY015-BD02
Prednisone Impurity 13
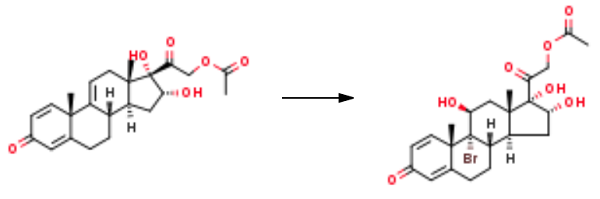
9α-bromo-11β,16α,17α,21-tetrahydroxypregnane-1,4-diene-3,20-dione-21-acetate
9α-bromo-11β,16α,17α,21-tetrahydroxypregnane-1,4-diene-3,20-dione-21-acetate
(11β,16α)-21-(Acetyloxy)-9-bromo-11,16,17-trihydroxy-pregna-1,4-diene-3,20-dione
Pregna-1,4-diene-3,20-dione, 21-(acetyloxy)-9-bromo-11,16,17-trihydroxy-, (11β,16α)- | [Molecular Formula]
C23H29BrO7 | [MOL File]
91160-89-3.mol | [Molecular Weight]
497.38 |
| Chemical Properties | Back Directory | [Boiling point ]
636.3±55.0 °C(Predicted) | [density ]
1.53±0.1 g/cm3(Predicted) | [pka]
11.57±0.70(Predicted) | [InChIKey]
LTRVYEKAPGRFOX-WFWLZCIZNA-N | [SMILES]
C[C@]12C[C@H](O)[C@@]3([C@]4(C=CC(=O)C=C4CC[C@@]3([H])[C@]1([H])C[C@@H](O)[C@]2(O)C(=O)COC(=O)C)C)Br |&1:1,3,5,6,15,17,20,22,r| |
| Hazard Information | Back Directory | [Uses]
(11β,16α)-21-(Acetyloxy)-9-bromo-11,16,17-trihydroxy-pregna-1,4-diene-3,20-dione is used as a reactant in the synthesis of triamcinolone acetonide, a synthetic corticosteroid used to treat skin conditions. | [Synthesis]
50.5 g of 2-((8S,10S,13S,14S,16R,17S)-16,17-dihydroxy-10,13-dimethyl-3-oxo-6,7,8,10,12,13,14,15,16,17-decahydro-3H-cyclopenta[a]phenanthren-17-yl)-2-oxoethyl acetate was dissolved in 1500 ml of acetone, 70 ml of 5% perchloric acid was added, the temperature was lowered to 0°C with stirring, and 45 g of N-bromosuccinimide was added and stirred After the reaction was completed, 50 ml of 20% sodium sulfite aqueous solution was added, the acetone was concentrated, the water was separated, filtered, and dried to obtain 62 g of Prednisone Impurity 13 (9α-bromo-11β,16α,17α,21-tetrahydroxypregnane-1,4-diene-3,20-dione-21-acetate).
|
|
|