| Identification | Back Directory | [Name]
4,7-DIMETHOXY-1,10-PHENANTHROLINE, 97% | [CAS]
92149-07-0 | [Synonyms]
4,7-Dimethoxy-1,1-phenanthroline
4,7-diMethoxyl-1,10-phenanthroline
4,7-DiMethoxy-1,10-phenanthroline 97%
4,7-DIMETHOXY-1,10-PHENANTHROLINE, 97%
4,7-Dimethoxy-1,10-phenanthroline ,98%
[4,7-DIMETHOXY-1,10-PHENANTHROLINE, 97%] | [Molecular Formula]
C14H12N2O2 | [MDL Number]
MFCD00233883 | [MOL File]
92149-07-0.mol | [Molecular Weight]
240.257 |
| Chemical Properties | Back Directory | [Melting point ]
197-212 °C | [Boiling point ]
373.1±37.0 °C(Predicted) | [density ]
1.25 | [storage temp. ]
Sealed in dry,2-8°C | [form ]
Crystalline Powder | [pka]
6.45±0.10(Predicted) | [color ]
White to brown | [Sensitive ]
air sensitive | [InChI]
InChI=1S/C14H12N2O2/c1-17-11-5-7-15-13-9(11)3-4-10-12(18-2)6-8-16-14(10)13/h3-8H,1-2H3 | [InChIKey]
ZPGVCQYKXIQWTP-UHFFFAOYSA-N | [SMILES]
N1C2C(=CC=C3C=2N=CC=C3OC)C(OC)=CC=1 |
| Hazard Information | Back Directory | [Chemical Properties]
White to brown Solid | [Uses]
Ligand for Cu-catalzyed N-arylation of imidazoles | [reaction suitability]
reagent type: ligand |
| Safety Data | Back Directory | [Hazard Codes ]
Xn,N | [Risk Statements ]
22-41-50 | [Safety Statements ]
26-39-61 | [RIDADR ]
UN 3077 9/PG 3 | [WGK Germany ]
3 | [TSCA ]
No | [HazardClass ]
9 | [PackingGroup ]
Ⅲ | [HS Code ]
29339900 | [Storage Class]
11 - Combustible Solids | [Hazard Classifications]
Acute Tox. 4 Oral
Aquatic Acute 1
Eye Dam. 1 |
| Questions And Answer | Back Directory | [Reaction]
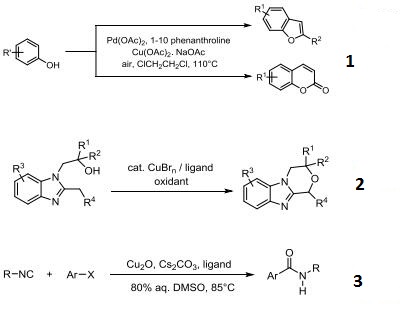
- Palladium-catalyzed synthesis of benzofurans and coumarins from phenols and olefins.
- Copper-catalyzed intermolecular amidation and imidation of unactivted alkanes.
- Synthesis of amides via copper-catalyzed amidation of aryl halides using isocyanides.
- Copper-catalyzed benzylic C(sp3)-H alkoxylation of heterocyclic compounds.
- Iridium-catalyzed silylation of aryl C-H bonds.
- Palladium-catalyzed intramolecular cyclization of nitroalkenes: synthesis of thienopyrroles.
- A Copper-catalyzed N-alkynylation route to 2-substitued N-alkynyl pyrroles and their cyclization into pyrrolo[2,1-c]oxazin-1-ones


|
|
|