1000801-75-1
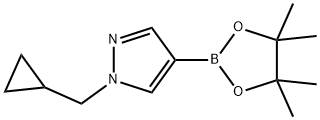 1000801-75-1 结构式
1000801-75-1 结构式
基本信息
1-(环丙基甲基)-吡唑-4-硼酸嚬哪醇酯
1-环丙基甲基-1H-吡唑-4-硼酸频哪醇酯
1-(环丙基甲基)-4-(4,4,5,5-四甲基-1,3,2-二硼烷-2-基)-1H-吡唑
1-(环丙基甲基)-4-(4,4,5,5-四甲基-1,3,2-二噁硼烷-2-基)-1H-吡唑
1-(Cyclopropylmethyl)pyrazole-4-boronic Acid Pinacol Ester
1-CyclopropylMethyl-1H-pyrazole-4-boronic acid, pinacol ester
1-Cyclopropylmethyl-1H-pyrazole-4-boronic acid, pinacol ester 97%
1-(cyclopropylmethyl)-4-(tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole
1-(cyclopropylmethyl)-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyrazole
1-(CyclopropylMethyl)-4-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole
1H-Pyrazole, 1-(cyclopropylMethyl)-4-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)-
物理化学性质
制备方法

269410-08-4

7051-34-5

1000801-75-1
以4-吡唑硼酸频哪醇酯和溴甲基环丙烷为原料合成1-(环丙基甲基)-4-(4,4,5,5-四甲基-1,3,2-二噁硼烷-2-基)-1H-吡唑的一般步骤:在0°C下,将溴甲基环丙烷(0.95 mg,6.70 mmol,0.70 mL,95%)加入到含有4-(4,4,5,5-四甲基-1,3,2-二氧杂硼杂环戊烷-2-基)-1H-吡唑(1.00 g,5.15 mmol)和碳酸铯(3.49 mg,10.72 mmol)的无水N,N-二甲基甲酰胺(20 mL)混合物中。搅拌30分钟后,移除冰水浴。将反应混合物在室温下搅拌过夜。随后,用乙酸乙酯(150 mL)稀释反应混合物,并用盐水(3×100 mL)洗涤。有机层用硫酸钠干燥,真空浓缩后得到1.30 g(4.38 mmol,产率85%)目标化合物1-(环丙基甲基)-4-(4,4,5,5-四甲基-1,3,2-二噁硼烷-2-基)-1H-吡唑。GC-MS(方法L9):Rt = 4.35 min; m/z = 247。1H NMR(300 MHz,CDCl3,方法M2)δ 7.81(s,1H),7.79(s,1H),3.99(d,J = 7.1 Hz,2H),1.32(s,12H),1.27(m,1H),0.71-0.58(m,2H),0.41-0.33(m,2H)。
参考文献:
[1] Journal of Medicinal Chemistry, 2014, vol. 57, # 10, p. 4196 - 4212
[2] Patent: WO2017/178416, 2017, A1. Location in patent: Page/Page column 114; 115
[3] Patent: WO2014/210354, 2014, A1. Location in patent: Page/Page column 47; 48
[4] Patent: WO2017/102091, 2017, A1. Location in patent: Page/Page column 435
[5] Patent: WO2017/207534, 2017, A1. Location in patent: Page/Page column 180
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | XW02100080175102 | 1-(环丙基甲基)-4-(4,4,5,5-四甲基-1,3,2-二噁硼烷-2-基)-1H-吡唑 | 1000801-75-1 | 250MG | 91元 |
| 2025/12/22 | XW02100080175101 | 1-(环丙基甲基)-4-(4,4,5,5-四甲基-1,3,2-二噁硼烷-2-基)-1H-吡唑 | 1000801-75-1 | 100MG | 65元 |
| 2025/05/22 | XW02100080175104 | 1-(环丙基甲基)-4-(4,4,5,5-四甲基-1,3,2-二噁硼烷-2-基)-1H-吡唑 | 1000801-75-1 | 5G | 1301元 |