1007881-98-2
 1007881-98-2 结构式
1007881-98-2 结构式
基本信息
(S)-叔丁基2-(2-(4-溴苯基)-2-乙羰基甲酰胺基)吡咯烷-1-羧酸酯
(S)-2-((2-(4-溴苯基)-2-氧乙基)氨基甲酰基)吡咯烷-1-羧酸叔丁酯
(S)-2-((2-(4-溴苯基)-2-氧代乙基)氨基甲酰基)吡咯烷-1-羧酸叔丁酯
(S)-叔丁基2-(2-(4-溴苯基)-2-乙羰基甲酰胺基)吡咯烷-1-羧酸酯(南3)
1,1-二甲基乙基 (2S)-2-({[2-(4-溴苯基)-2-氧代乙基]氨基}羰基)-1-吡咯烷羧酸
(S)-tert-butyl 2-(2-(4-bromophenyl)-2-oxoethylcarbamoyl)pyrrolidine-1-carboxylate
tert-Butyl (S)-2-((2-(4-bromophenyl)-2-oxoethyl)carbamoyl)pyrrolidine-1-carboxylate
tert-butyl (2S)-2-[[2-(4-bromophenyl)-2-oxoethyl]carbamoyl]pyrrolidine-1-carboxylate
(S)-2-[2-(4-Bromo-phenyl)-2-oxo-ethylcarbamoyl]-pyrrolidine-1-carboxylic acid tert-butyl ester
1-Pyrrolidinecarboxylic acid, 2-[[[2-(4-bromophenyl)-2-oxoethyl]amino]carbonyl]-, 1,1-dimethylethyl ester, (2S)-
制备方法
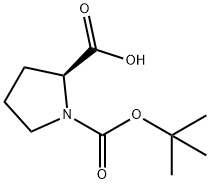
15761-39-4
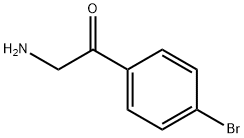
7644-04-4

1007881-98-2
以BOC-L-脯氨酸和2-氨基-4'-溴苯乙酮为原料合成(S)-2-((2-(4-溴苯基)-2-氧代乙基)氨基甲酰基)吡咯烷-1-羧酸叔丁酯的一般步骤:在干燥的DMF(600 mL)中,依次加入2-氨基-1-(4-溴苯基)乙酮(50 g,0.2 mol)、BOC-L-脯氨酸(43.0 g,0.2 mol)、HATU(53 g,0.21 mol)和N,N-二异丙基乙胺(80.0 g,0.62 mol)。将反应混合物在5℃下搅拌1小时。反应完成后,减压蒸馏去除大部分DMF,将残余物溶于乙酸乙酯(600 mL)中,并用饱和NaHCO3水溶液(500 mL)和盐水(500 mL)依次洗涤。有机层经无水MgSO4干燥,过滤后减压浓缩。粗产物通过硅胶柱色谱纯化,洗脱剂为石油醚/乙酸乙酯(3:1至1:1),得到浅黄色固体中间体I-11(60 g,收率62%)。1H NMR (400 MHz, CDCl3) δ: 7.85 (d, J = 8.4 Hz, 2H), 7.66 (d, J = 8.4 Hz, 2H), 4.67-4.80 (m, 2H), 4.33-4.41 (m, 1H), 3.42-3.53 (m, 2H), 2.19-2.31 (m, 2H), 1.90-2.00 (m, 2H), 1.50 (s, 9H), 1.10。
参考文献:
[1] Patent: WO2011/15657, 2011, A1. Location in patent: Page/Page column 20
[2] Patent: WO2011/54834, 2011, A1. Location in patent: Page/Page column 31-32
[3] Patent: US2012/136027, 2012, A1. Location in patent: Page/Page column 9
[4] Journal of Medicinal Chemistry, 2014, vol. 57, # 5, p. 2058 - 2073
