1010120-55-4
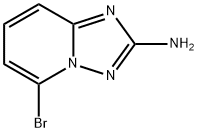 1010120-55-4 结构式
1010120-55-4 结构式
基本信息
5-溴-[1,2,4]三噻唑[1,5-A]吡啶-2-胺
5-溴-2-氨基[1,2,4]三唑[1,5-A]并吡啶
2-氨基-5-溴-[1,2,4]三唑并[1,5-A]吡啶
5-溴-[1,2,4]三氮唑并[1,5-A]吡啶-2-基胺
2-Amino-5-bromo-1,2,4-triazolo[1,5-a]pyridine
5-bromo-[1,2,4]triazolo[1,5-a]pyridin-2-amine
1,2,4]Triazolo[1,5-a]pyridin-2-amine, 5-bromo-
5-Bromo-[1,2,4]triazolo[1,5-a]pyridin-2-ylamine
5-bromo-[1,2,4]trithiazole[1,5-A]pyridin-2-amine
5-Bromo-[1,2,4] triazolo[1,5-a]pyridine-2-ylamine
5-Bromo-[1,2,4]triazolo[1,5-a]pyridin-2-ylamine manufacturers
物理化学性质
制备方法

1010120-59-8
![5-溴-[1,2,4]三噻唑[1,5-A]吡啶-2-胺](/CAS2/GIF/1010120-55-4.gif)
1010120-55-4
以(6-溴吡啶-2-氨基)碳硫酰氨基甲酸乙酯为原料合成5-溴-[1,2,4]三唑并[1,5-a]吡啶-2-胺的一般步骤:将[(6-甲基吡啶-2-基)氨基甲酰基]氨基甲酸乙酯(43.7 g,0.144 mmol,1当量)、盐酸羟胺(51.3 g,0.738 mmol,5.13当量)和二异丙基乙胺(75.2 mL,0.432 mmol,3.00当量)溶于1:1甲醇/乙醇混合溶剂(500 mL)中,加热至70℃并保持3.5小时。反应完成后,将混合物冷却至24℃,过滤收集所得固体。用冷水(2×100 mL)洗涤固体,然后在真空(约1 mmHg)下干燥,得到白色固体产物(24.4 g,收率79.5%)。产物经1H NMR(400 MHz,DMSO-d6)表征,化学位移δ:7.32-7.38(m,2H),7.21(m,1H),6.23(br s,2H)。
参考文献:
[1] Bioorganic and Medicinal Chemistry Letters, 2013, vol. 23, # 17, p. 5014 - 5021
[2] Patent: WO2009/155565, 2009, A1. Location in patent: Page/Page column 92-93
[3] Patent: WO2012/970, 2012, A1. Location in patent: Page/Page column 30-31
[4] Journal of Medicinal Chemistry, 2017, vol. 60, # 13, p. 5663 - 5672
[5] Patent: WO2010/141796, 2010, A2. Location in patent: Page/Page column 182
