1014613-64-9
 1014613-64-9 结构式
1014613-64-9 结构式
基本信息
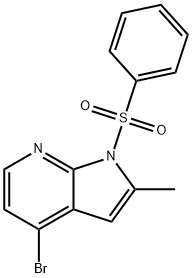
4-溴-2-甲基-1氢-吡咯并吡啶
4-溴-2-甲基-1H-吡咯并[2,3-B]吡啶
1H-Pyrrolo[2,3-b]pyridine, 4-broMo-2-Methyl-
4-Bromo-2-methyl-1H-pyrrolo[2,3-b]pyridine 95%
(2R,3R,4R,5R)-2-(Acetoxymethyl)-5-(6-amino-9H-purin-9-yl)tetrahydrofuran-3,4-diyl diacetate
物理化学性质
制备方法
1014613-05-8
![4-溴-2-甲基-1H-吡咯并[2,3-B]吡啶](/CAS/GIF/1014613-64-9.gif)
1014613-64-9
以4-溴-2-甲基-1-(苯磺酰基)-1H-吡咯并[2,3-b]吡啶为原料合成4-溴-2-甲基-1H-吡咯并[2,3-b]吡啶的一般步骤:向4-溴-2-甲基-1-(苯磺酰基)-1H-吡咯并[2,3-b]吡啶(16g,0.045mol)的1,4-二恶烷(320mL)溶液中加入2M氢氧化钠水溶液(114mL,0.228mol)。将反应混合物加热至60℃,保持16小时。反应完成后,将混合物冷却至室温。分离有机层,水层用乙酸乙酯萃取。合并有机相,用饱和食盐水洗涤,无水硫酸镁干燥,过滤后减压浓缩,得到4-溴-2-甲基-1H-吡咯并[2,3-b]吡啶,为乳白色固体(10.07g,产率104%)。LC/MS(方法B):m/z 211/213 [M+H]+,保留时间1.04分钟。
参考文献:
[1] Patent: WO2009/112475, 2009, A1. Location in patent: Page/Page column 62
[2] Bioorganic and Medicinal Chemistry Letters, 2009, vol. 19, # 9, p. 2504 - 2508
[3] Patent: US2010/160647, 2010, A1. Location in patent: Page/Page column 21
[4] Patent: WO2012/170752, 2012, A1. Location in patent: Page/Page column 42
[5] Bioorganic and Medicinal Chemistry, 2015, vol. 23, # 15, p. 4344 - 4353
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | XW02738757703 | 2'',3'',5''-三乙酰腺苷 (2R,3R,4R,5R)-2-(Acetoxymethyl)-5-(6-amino-9H-purin-9-yl)tetrahydrofuran-3,4-diyl diacetate | 1014613-64-9 | 100g | 463元 |
| 2025/12/22 | XW02101461364905 | 4-溴-2-甲基-1H-吡咯并[2,3-B]吡啶 | 1014613-64-9 | 10G | 4231元 |
| 2025/12/22 | XW02101461364904 | 4-溴-2-甲基-1H-吡咯并[2,3-B]吡啶 | 1014613-64-9 | 5G | 2116元 |

