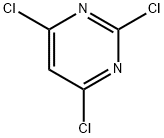
10397-13-4
 10397-13-4 结构式
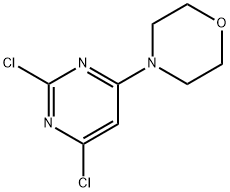
10397-13-4 结构式
基本信息
2-(4-吗啉基)-4
4,6-二氯-2-吗啉基嘧啶
2-吗啡林-4,6-二氯嘧啶
2-吗啉基-4,6-二氯嘧啶
2-吗啡啉基-4,6-二氯嘧啶
4,6-二氯-2-吗啡啉基嘧啶
4-(4,6-二氯嘧啶-2-基)吗啉
2-(4-吗啉基)-4,6-二氯嘧啶
4-(4,6-二氯-2-嘧啶基)吗啉
2-Morpholino-4,6-dichloropyrimidine
4,6-dichloro-2-MorpholinopyriMidine
4-(4,6-Dichlorpyrimidin-2-yl)morpholin
4-(4,6-Dichloro-2-pyriMidyl)Morpholine
2-(4-Morpholino)-4,6-dichloropyrimidine
4-(4,6-Dichloropyrimidin-2-yl)morpholine
4-(4,6-Dichloro-2-pyrimidyl)morpholine>
Morpholine, 4-(4,6-dichloro-2-pyrimidinyl)-
4-(4,6-Dichloro-2-pyriMidinyl)Morpholine, 97%
物理化学性质
制备方法

110-91-8
3764-01-0

10397-13-4
52127-83-0
将2,4,6-三氯嘧啶(5)(3.158g,17.22mmol)与丙酮(60mL)混合,并将混合物冷却至0℃。在0℃下缓慢加入吗啉(7)(1.05当量,1.576g,18.09mmol),随后在0℃搅拌反应混合物15分钟,然后升温至室温继续搅拌15分钟。反应进程通过薄层色谱法(TLC,展开剂为20%乙酸乙酯的己烷溶液)进行监测。反应完成后,通过旋转蒸发仪浓缩反应混合物,并在高真空下进一步干燥。粗产物通过硅胶柱色谱法纯化,使用20%乙酸乙酯的己烷溶液作为洗脱剂,得到主要区域异构体2-吗啉基-4,6-二氯嘧啶(10)(3.183g,13.6mmol,收率79%)。元素分析(C8H9N3OCl2)计算值:C,41.05;H,3.88;实测值:C,42.27;H,4.06。1H-NMR(300MHz,CDCl3)δ6.40(s,1H),3.82-3.70(m,4H),3.65(m,4H)。高分辨质谱(HRMS)[M]+计算值:C8H9N3OCl2,234.0201;实测值:234.0196。次要区域异构体4-(2,6-二氯嘧啶-4-基)吗啉(11)(0.806g,3.444mmol,收率20%)。1H-NMR(300MHz,CDCl3)δ6.56(s,1H),3.85-3.60(m,8H)。13C-NMR(101MHz,CDCl3)δ161.75,160.55,108.31,66.59,44.39。HRMS [M]+计算值:C8H9N3OCl2,234.0201;实测值:234.0196。
参考文献:
[1] Molecules, 2018, vol. 23, # 7, p. 1 - 13
[2] Patent: WO2015/49369, 2015, A1. Location in patent: Page/Page column 48; 49
[3] Patent: WO2016/75130, 2016, A1. Location in patent: Page/Page column 124-125
[4] Patent: WO2017/198347, 2017, A1. Location in patent: Page/Page column 88; 89
[5] Patent: WO2017/198346, 2017, A1. Location in patent: Page/Page column 77; 78
