1057682-03-7
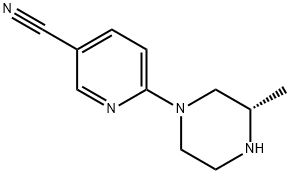 1057682-03-7 结构式
1057682-03-7 结构式
基本信息
Ethanone,1-[5-bromo-3-(trifluoromethyl)-7-pyridinyl]-
6-[(3S)-3-methylpiperazin-1-yl]pyridine-3-carbonitrile
6-[(3S)-3-Methyl-1-piperazinyl]-3-pyridinecarbonitrile
3-Pyridinecarbonitrile,6-[(3S)-3-methyl-1-piperazinyl]-
(S)-6-(3-methylpiperazin-1-yl)nicotinonitrile1057682-03-7
物理化学性质
制备方法
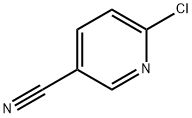
33252-28-7

74879-18-8

1057682-03-7
以2-氯-5-氰基吡啶和(S)-2-甲基哌嗪为原料合成(S)-6-(3-甲基哌嗪-1-基)烟腈的一般步骤:向含有6-氯烟腈(1.38g,10mmol,1当量)和(S)-2-甲基哌嗪(1.00g,10mmol)的DMF(15mL)溶液中加入三乙胺(4.13g,3mL,40.8mmol,4当量)。将反应混合物在室温下搅拌14小时,期间观察到白色三乙胺盐酸盐沉淀的形成。反应完成后,加入水(15mL)和乙酸乙酯(100mL)进行萃取,分离有机层,用无水硫酸钠干燥,减压浓缩得到白色残余物。将固体在高真空下进一步干燥,得到目标产物(S)-6-(3-甲基哌嗪-1-基)烟腈,为白色固体(1.4g,产率69%)。产物的1H NMR(400MHz,氯仿-d)数据如下:δ 8.38(s,1H),7.58(d,J = 9.60Hz,1H),6.59(d,J = 9.09Hz,1H),4.19-4.31(m,2H),3.08-3.15(m,1H),2.92-3.04(m,1H),2.81-2.91(m,2H),2.57-2.65(m,1H),1.15(d,J = 6.32Hz,3H)。
参考文献:
[1] Journal of Medicinal Chemistry, 2009, vol. 52, # 13, p. 3954 - 3968
[2] Patent: WO2008/110611, 2008, A1. Location in patent: Page/Page column 34
[3] Patent: WO2018/125961, 2018, A1. Location in patent: Page/Page column 110; 113
