106961-33-5
 106961-33-5 结构式
106961-33-5 结构式
基本信息
N,N,6-三甲基-2-(4-甲基苯基)咪唑并[1,2-Α]吡啶-3-甲胺
N,N-二甲基-1-(6-甲基-2-(对甲苯)咪唑并[1,2-A]吡啶-3-基)甲胺
Zolpidem Impurity 67
imidazo[1,2-a]pyridin-3-yl)
N,N-Dimethyl-1-(6-methyl-2-(p-tolyl)
DIMETHYL-(6-METHYL-2-P-TOLYL-IMIDAZO[1,2-A]PYRIDIN-3-YLMETHYL)-AMINE
Imidazo[1,2-a]pyridine-3-methanamine, N,N,6-trimethyl-2-(4-methylphenyl)-
3-(DiMethylaMinoMethyl)-6-Methyl-2-(4-Methylphenyl)iMidazo[1,2-a]pyridine
N,N-dimethyl-1-(6-methyl-2-(p-tolyl)imidazo[1,2-a]pyridin-3-yl)methanamine
物理化学性质
制备方法
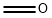
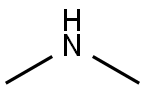
50-00-0
![6-甲基-2-(4-甲基苯基)咪唑[1,2-a]吡啶](/CAS/GIF/88965-00-8.gif)
88965-00-8
124-40-3
![N,N,6-三甲基-2-(4-甲基苯基)咪唑并[1,2-Α]吡啶-3-甲胺](/CAS/GIF/106961-33-5.gif)
106961-33-5
步骤(i):制备N,N-二甲基-1-(6-甲基-2-(对甲苯基)咪唑并[1,2-a]吡啶-3-基)甲胺(8)。在搅拌下,将6-甲基-2-(4-甲基苯基)咪唑并[1,2-a]吡啶(200克,0.92摩尔)溶解于乙酸(985毫升)中。将反应混合物冷却至0-5℃,缓慢加入40%二甲胺水溶液(157克,1.39摩尔),随后加入多聚甲醛(36.11克,1.20摩尔)。在50-55℃下搅拌反应混合物3-4小时,随后在减压下除去乙酸。加入水(1.5升),通过硅藻土垫过滤,并通过滴加30%氢氧化钠溶液将滤液碱化至pH 8.0-8.5。过滤得到灰白色固体,依次用水(500毫升)和己烷(200毫升)洗涤。将固体在烘箱中干燥至恒重,得到N,N-二甲基-1-(6-甲基-2-(对甲苯基)咪唑并[1,2-a]吡啶-3-基)甲胺(8)230克,产率91.6%,纯度93.5%。熔点范围:137.9-141.5℃。IR光谱(cm^-1):2944, 2809, 2761, 1502, 1454, 1389, 1018, 829, 799。1H NMR(400 MHz, CDCl3):δ 2.25(s, 6H), 2.36(s, 3H), 2.40(s, 3H), 3.84(s, 2H), 7.04(dd, J = 9.1 Hz, J = 1.6 Hz, 1H), 7.25(d, J = 7.9 Hz, 2H), 7.52(d, J = 9.1 Hz, 1H), 7.69(d, J = 8.0 Hz, 2H), 8.10(s, 1H)。质谱(m/z):280.4(M + H)+。
参考文献:
[1] Patent: WO2009/7995, 2009, A1. Location in patent: Page/Page column 11; 13-14; 15-17
[2] Farmaco, 1991, vol. 46, # SUPPL. 1, p. 277 - 288
[3] Acta Poloniae Pharmaceutica - Drug Research, 2001, vol. 58, # 1, p. 43 - 52
[4] Patent: US2007/27180, 2007, A1. Location in patent: Page/Page column title page; sheet 1; 6