1071433-01-6
中文名称
METHYL 2-METHYLINDAZOLE-6-CARBOXYLATE
英文名称
Methyl 2-methylindazole-6-carboxylate
CAS
1071433-01-6
分子式
C10H10N2O2
分子量
190.2
MOL 文件
1071433-01-6.mol
更新日期
2023/03/20 15:41:21
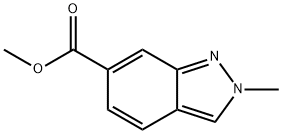 1071433-01-6 结构式
1071433-01-6 结构式
基本信息
中文别名
2-甲基-2H-吲唑-6-羧酸甲酯METHYL 2-METHYL-2H-INDAZOLE-6-CARBOXYLATE 2-甲基-2H-吲唑-6-羧酸甲酯 CAS:1071433-01-6
英文别名
-2H-indazoLMethyl 2-methylindazole-6-carboxylate
Methyl 2-Methyl-2H-indazol-6-carboxylate
6-(Methoxycarbonyl)-2-methyl-2H-indazole
methyl 2-methyl-2H-indazole-6-carboxylate
2-Methyl-2H-indazole-6-carboxylic acid methyl ester
2H-Indazole-6-carboxylic acid, 2-methyl-, methyl ester
物理化学性质
沸点338.9±15.0 °C(Predicted)
密度1.23±0.1 g/cm3(Predicted)
储存条件Sealed in dry,Room Temperature
酸度系数(pKa)-0.39±0.30(Predicted)
形态solid
外观Off-white to light yellow Solid
InChI1S/C10H10N2O2/c1-12-6-8-4-3-7(10(13)14-2)5-9(8)11-12/h3-6H,1-2H3
InChIKeyDPDPLMPLBWKMSG-UHFFFAOYSA-N
SMILESCOC(=O)c1ccc2cn(C)nc2c1
制备方法
方法1

170487-40-8
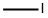
74-88-4
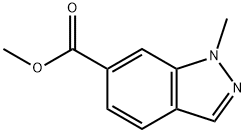
1007219-73-9

1071433-01-6
在冰浴冷却条件下,向1H-吲唑-6-羧酸甲酯(566 mg,3.21 mmol)的无水四氢呋喃溶液中分批加入氢化钠(60%分散于矿物油中,154 mg,3.85 mmol)。将反应混合物于室温下搅拌30分钟。随后,缓慢滴加碘甲烷(547 mg,3.85 mmol),继续室温搅拌反应过夜。反应完成后,将混合物冷却至0℃,小心加入水淬灭反应,并用乙酸乙酯萃取。合并有机相,经无水硫酸钠干燥后,减压浓缩。通过硅胶柱色谱法纯化,得到1-甲基-1H-吲唑-6-羧酸甲酯(130 mg)和2-甲基-2H-吲唑-6-羧酸甲酯(230 mg),总收率为59%。产物经1H NMR确认结构:1-甲基-1H-吲唑-6-羧酸甲酯:1H NMR (400 MHz, CDCl3) δ 3.97 (3H, s), 4.14 (3H, s), 7.74-7.82 (2H, m), 8.02 (1H, s), 8.17 (1H, d, J = 0.8 Hz)。2-甲基-2H-吲唑-6-羧酸甲酯:1H NMR (400 MHz, CDCl3) δ 3.94 (3H, s), 4.25 (3H, s), 7.65-7.72 (2H, m), 7.92 (1H, s), 8.47 (1H, d, J = 1.2 Hz)。质谱分析:[M + H]+ m/z 191.0815 (计算值 C10H11N2O2, 191.0816)。
参考文献:
[1] Patent: WO2014/89364, 2014, A1. Location in patent: Paragraph 00477; 00478
