1095708-32-9
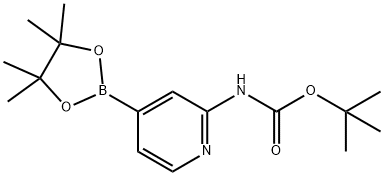 1095708-32-9 结构式
1095708-32-9 结构式
基本信息
2-(叔丁氧羰基氨基)吡啶-4-硼酸频哪醇酯
2-(叔丁氧基羰基氨基)吡啶-4-硼酸频那醇酯
2-(tert-ButoxycarbonylaMino)pyridine-4-boronic acid pinacol ester
(2-((TERT-BUTOXYCARBONYL)AMINO)PYRIDIN-4-YL)BORONIC ACID PINACOL ESTER
tert-butyl N-[4-(tetramethyl-1,3,2-dioxaborolan-2-yl)pyridin-2-yl]carbamate
N-tert-butyl-N-[3-(tetraMethyl-1,3,2-dioxaborolan-2-yl)pyridin-2-yl]carbaMate
tert-Butyl[4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridin -2-yl]carbamate
tert-butyl N-[4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridin-2-yl]carbamate
tert-butyl (4-(hydroxy((3-hydroxy-2,3-dimethylbutan-2-yl)oxy)boranyl)pyridin-2-yl)carbamate
[4-(4,4,5,5-Tetramethyl-[1,3,2]dioxaborolan-2-yl)-pyridin-2-yl]-carbamic acid tert-butyl ester 97%
Carbamic acid, N-[4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-2-pyridinyl]-, 1,1-dimethylethyl ester
物理化学性质
制备方法

207799-10-8
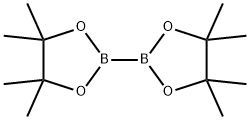
73183-34-3

1095708-32-9
以2-Boc胺-4-溴吡啶(0.494 g,1.81 mmol)、联硼酸频那醇酯(1.4 g,5.51 mmol)和乙酸钾(0.535 g,5.45 mmol)为原料,在二甲基亚砜(8 mL)中制备反应混合物。将混合物用氮气吹扫10分钟以除氧。随后加入[1,1'-双(二苯基膦基)二茂铁]二氯化钯(II)(0.150 g,0.184 mmol),再次用氮气吹扫5分钟。在氮气保护下,将反应混合物加热至85℃并搅拌1小时。反应完成后,将混合物用乙酸乙酯(15 mL)稀释,并用0.2 M盐酸水溶液(10 mL)洗涤。注意部分产物可能存在于水层中。小心地用饱和碳酸钠水溶液调节水层pH至约6,然后用乙酸乙酯(10 mL)萃取水层中的产物。合并有机层,用无水硫酸钠干燥,过滤后真空浓缩,得到2-(Boc-氨基)吡啶-4-硼酸频哪醇酯,为白色固体(0.44 g,产率76%)。产物经1H NMR(DMSO-d6)和LCMS表征:1H NMR (DMSO-d6) δ 1.29 (s, 12H), 1.45 (s, 9H), 7.15-7.17 (d, 1H), 8.07 (s, 1H), 8.24-8.25 (d, 1H), 9.77 (s, 1H); LCMS Rt = 0.68 min, MS m/z 183.1 [M+H]+。
参考文献:
[1] Journal of Medicinal Chemistry, 2017, vol. 60, # 13, p. 5613 - 5637
[2] Patent: WO2012/4714, 2012, A2. Location in patent: Page/Page column 62
[3] Patent: US2014/194443, 2014, A1. Location in patent: Paragraph 0543-0545
[4] Patent: WO2014/108337, 2014, A1. Location in patent: Page/Page column 69; 70
[5] Patent: US2010/179154, 2010, A1. Location in patent: Page/Page column 29
