116247-92-8
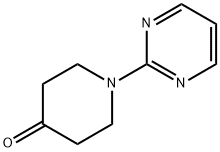 116247-92-8 结构式
116247-92-8 结构式
基本信息
1-(嘧啶-2基)哌啶-4-酮
1-(2-嘧啶基)四氢-4(1H)-吡啶酮
1-(2-pyrimidinyl)-2-piperidinone
4-Oxo-1-(pyrimidin-2-yl)piperidine
4-Piperidinone, 1-(2-pyrimidinyl)-
1-Pyrimidin-2-yl-piperidin-4-one95%
1-PYRIMIDIN-2-YL-PIPERIDIN-4-ONE 95%
1-(2-PYRIMIDINYL)TETRAHYDRO-4(1H)-PYRIDINONE
1-PYRIMIDIN-2-YL-PIPERIDIN-4-ONE ISO 9001:2015 REACH
4-Oxo-1-(pyrimidin-2-yl)piperidine, 2-(4-Oxopiperidin-1-yl)pyrimidine
物理化学性质
制备方法
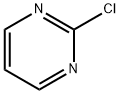
1722-12-9

41979-39-9

116247-92-8
以2-氯嘧啶和4-氧代哌啶酮盐酸盐为原料,合成1-(嘧啶-2-基)哌啶-4-酮的一般步骤如下: 5.1 1-(嘧啶-2-基)哌啶-4-酮的制备: A. 将4-哌啶酮一水合物盐酸盐(4.84 g,31.5 mmol)、2-氯嘧啶(3.44 g,30 mmol)和三乙胺(TEA,10.04 mL,72 mmol)的混合物溶于乙醇(EtOH,150 mL)中,加热回流过夜。反应完成后,将混合物浓缩至近干,并用乙酸乙酯(EtOAc,400 mL)稀释。依次用水(2×50 mL)和盐水(2×50 mL)洗涤EtOAc层。水层用EtOAc(4×100 mL)反萃取。合并所有EtOAc层,用无水硫酸钠(Na2SO4)干燥,过滤后减压浓缩溶剂。残余物通过ISCO快速色谱法纯化(120 g硅胶柱,洗脱程序:己烷5分钟,0-80% EtOAc/己烷梯度洗脱70分钟,最后纯EtOAc洗脱15分钟),得到目标产物1-(嘧啶-2-基)哌啶-4-酮(3.5 g,收率66%)。 产物表征数据如下: HPLC分析条件:Luna Phenyl-Hexyl 5μm 4.6×50 mm色谱柱,流动相A为10 mM乙酸铵水溶液,流动相B为乙腈,梯度洗脱(10-90% B,3分钟),流速3 mL/min,保留时间0.97和1.08分钟。 质谱(MS):m/z 178 [M+H]+。 1H NMR (300 MHz, CDCl3): δ 2.52 (t, J = 6.29 Hz, 4H), 4.15 (t, J = 6.20 Hz, 4H), 6.60 (t, J = 4.67 Hz, 1H), 8.38 (d, J = 4.77 Hz, 2H)。
参考文献:
[1] Patent: WO2008/58064, 2008, A1. Location in patent: Page/Page column 17
[2] Patent: WO2008/58068, 2008, A1. Location in patent: Page/Page column 23
[3] Patent: WO2008/67120, 2008, A2. Location in patent: Page/Page column 23
[4] Patent: WO2008/67121, 2008, A2. Location in patent: Page/Page column 52
[5] Patent: US2006/258672, 2006, A1. Location in patent: Page/Page column 17
