1172623-99-2
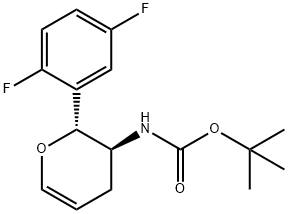
 1172623-99-2 结构式
1172623-99-2 结构式
基本信息
奥格列汀中间体 MK-3102中间体
奥格列汀中间体 MK-3102中间体 200G
(5S,6R)-5-(BOC-氨基)-6-(2,5-二氟苯基)-3-羟基四氢吡喃
((2R,3S)-2-(2,5-二氟苯基)-5-羟基四氢-2H-吡喃-3-基)氨基甲酸叔丁酯
(2XI,5R)-1,5-脱水-3,4-二脱氧-5-C-(2,5-二氟苯基)-4-[[叔丁氧羰基]氨基]-D-甘油型戊糖醇
(5S,6R)-5-(Boc-amino)-6-(2,5-difluorophenyl)-3-hydroxytetrahydropyran
tert-butyl N-[(2R,3S)-2-(2,5-difluorophenyl)-5-hydroxyoxan-3-yl]carbamate
tert-butyl [(2R,3S)-2-(2,5-difluorophenyl)-5-hydroxytetrahydro-2H-pyran-3-yl]carbaMate
tert-butyl [(2R,3S)-2-(2,5-difluorophenyl)-5-hydroxytetrahydro-2H-pyran-3-yl]carbaMate Synonyms
(2xi,5R)-1,5-Anhydro-3,4-dideoxy-5-C-(2,5-difluorophenyl)-4-[[(1,1-dimethylethoxy)carbonyl]amino]-D-glycero-pentitol
D-glycero-Pentitol, 1,5-anhydro-3,4-dideoxy-5-C-(2,5-difluorophenyl)-4-[[(1,1-dimethylethoxy)carbonyl]amino]-, (2ξ,5R)-
物理化学性质
制备方法
1172623-98-1

1172623-99-2
以((2R,3S)-2-(2,5-二氟苯基)-3,4-二氢-2H-吡喃-3-基)氨基甲酸叔丁酯(8.9g,28.6mmol)为原料,溶于无水甲基叔丁基醚(90mL)中,加入无水甲苯(9mL),将反应体系降温至-10℃。缓慢加入硼烷二甲基硫醚的四氢呋喃溶液(2mol/L,35.9mL),保持反应温度在0℃下反应3.5小时。反应完成后,缓慢加入水(4mL),随后滴加氢氧化钠溶液(1mol/L,89mL),搅拌15分钟。分批加入过硼酸钠(13.2g,85.8mmol),室温下搅拌过夜。反应完成后,将反应液静置分层,水相用甲基叔丁基醚(50mL×2)萃取。合并有机相,用饱和氯化钠溶液(20mL×2)洗涤,无水硫酸钠干燥,过滤后浓缩。向浓缩物中加入甲苯(50mL),加热至90℃溶解。缓慢滴加正己烷(200mL)至反应液中,析出白色固体,过滤。滤饼用正己烷(30mL×2)洗涤,浓缩除去溶剂,得到白色固体粉末目标产物((2R,3S)-2-(2,5-二氟苯基)-5-羟基四氢-2H-吡喃-3-基)氨基甲酸叔丁酯(7.9g,收率84%)。质谱(ESI)m/z:274.1 [M-55]。
参考文献:
[1] Organic Process Research and Development, 2015, vol. 19, # 11, p. 1760 - 1768
[2] Patent: EP3159344, 2017, A1. Location in patent: Paragraph 0096; 0097; 0104
[3] Patent: TW2017/8221, 2017, A. Location in patent: Page/Page column 33; 36
[4] Patent: TW2017/8224, 2017, A. Location in patent: Page/Page column 28; 32
[5] Patent: TW2017/8220, 2017, A. Location in patent: Page/Page column 56; 57; 60
