1225278-64-7
 1225278-64-7 结构式
1225278-64-7 结构式
基本信息
1,3-Dioxane-5,5-dimethanol,2-(6-methoxyphenyl)-
2-chloro-4-(2,5-difluoro-4-nitrophenoxy)pyridine
Pyridine,2-chloro-4-(2,5-difluoro-4-nitrophenoxy)-
制备方法

17368-12-6
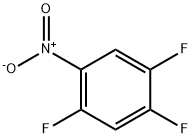
2105-61-5

1225278-64-7
在氩气保护下,将氢化钠(136 mg,3.4 mmol,60%矿物油分散体)分批加入2-氯-4-羟基吡啶(2 g,15.4 mmol)的无水N,N-二甲基甲酰胺(DMF,38 mL)溶液中。反应混合物在0℃下搅拌1小时。随后,滴加2,4,5-三氟硝基苯(626 mg,3.1 mmol)的无水DMF(7.6 mL)溶液,并将反应体系在氩气保护下升温至90℃持续搅拌3小时。反应完成后,将混合物冷却至室温并继续搅拌过夜。减压蒸馏除去溶剂,粗产物用50 mL水和50 mL乙酸乙酯(EtOAc)分配。水相用乙酸乙酯(3×50 mL)萃取,合并有机相后用饱和食盐水洗涤,无水硫酸钠干燥。减压浓缩后,通过硅胶柱色谱法(洗脱剂:正己烷/乙酸乙酯梯度)纯化,得到目标化合物2-氯-4-(2,5-二氟-4-硝基苯氧基)吡啶(3.57 g,收率81%)。产物经1H NMR(400 MHz,DMSO-d6)和质谱(ESI)表征:1H NMR δ 8.43-8.33 (m, 2H), 7.85-7.79 (m, 1H), 7.33 (d, 1H), 7.20-7.18 (m, 1H); MS (ESI) m/z: 287.0 [M+H]+。
参考文献:
[1] Patent: WO2010/51373, 2010, A1. Location in patent: Page/Page column 73-74
[2] Patent: WO2011/137342, 2011, A1. Location in patent: Page/Page column 31
[3] Patent: WO2013/78295, 2013, A2. Location in patent: Paragraph 00171
[4] Patent: WO2014/145004, 2014, A1. Location in patent: Paragraph 0100
[5] Patent: US2014/315917, 2014, A1. Location in patent: Paragraph 0312