1257704-57-6
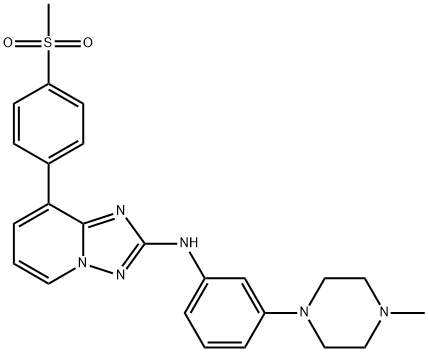 1257704-57-6 结构式
1257704-57-6 结构式
基本信息
N-[3-(4-甲基-1-哌嗪基)苯基]-8-[4-(甲磺酰基)苯基]-[1,2,4]三唑并[1,5-A]吡啶-2-胺
CEP 33779 USP/EP/BP
CEP33779
CEP-33779
CEP 33779.
N-(3-(4-Methylpiperazin-1-yl)phenyl)-8-(4-(methylsulfonyl)phenyl)-[1,2,4]triazolo[1,5-a]pyridi
N-[3-(4-methylpiperazin-1-yl)phenyl]-8-(4-methylsulfonylphenyl)-[1,2,4]triazolo[1,5-a]pyridin-2-amine
[8-(4-Methylsulfonylphenyl)[1,2,4]triazolo[1,5-a]pyridin-2-yl][3-(4-methylpiperazin-1-yl)phenyl]amine
N-[3-(4-Methyl-1-piperazinyl)phenyl]-8-[4-(methylsulfonyl)phenyl]-[1,2,4]triazolo[1,5-a]pyridin-2-amine
[1,2,4]Triazolo[1,5-a]pyridin-2-amine, N-[3-(4-methyl-1-piperazinyl)phenyl]-8-[4-(methylsulfonyl)phenyl]-
{[8-(4-methanesulfonyl-phenyl)-[1,2,4]triazolo[1,5-a]-pyridin-2-yl]-[3-(4-methyl-piperazin-1-yl)-phenyl]-amine}
物理化学性质
制备方法

747413-17-8
![[1,2,4]Triazolo[1,5-a]pyridin-2-amine, 8-[4-(methylsulfonyl)phenyl]-](/CAS/20210305/GIF/1257704-13-4.gif)
1257704-13-4
![N-[3-(4-甲基-1-哌嗪基)苯基]-8-[4-(甲磺酰基)苯基]-[1,2,4]三唑并[1,5-A]吡啶-2-胺](/CAS/20150408/GIF/1257704-57-6.gif)
1257704-57-6
一般步骤:向烘箱干燥的反应管中加入乙酸钯(10 mg)、2,2'-双(二环己基膦基)联苯(30 mg)、8-[4-(甲磺酰基)苯基]-[1,2,4]三唑并[1,5-a]吡啶-2-胺(75 mg)、1-(3-溴苯基)-4-甲基哌嗪(80 mg)、碳酸铯(270 mg)和1,4-二恶烷(5 mL)。将反应管抽真空并用氮气反冲三次,然后密封。将反应混合物在80℃下加热72小时。反应完成后,将混合物冷却至室温。用二氯甲烷(10 mL)稀释冷却的混合物,通过硅藻土塞过滤,并用二氯甲烷冲洗。滤液经蒸发浓缩后,通过柱色谱法纯化(使用ISCO自动纯化系统,胺改性的硅胶柱,洗脱剂为5%至100%乙酸乙酯的己烷溶液)。得到目标化合物N-[3-(4-甲基-1-哌嗪基)苯基]-8-[4-(甲磺酰基)苯基]-[1,2,4]三唑并[1,5-a]吡啶-2-胺(化合物A),为黄色固体(70 mg)。熔点:232-234℃。1H NMR (400 MHz, CDCl3, δ, ppm): 8.49 (d, J = 7.2 Hz, 1H), 8.25 (d, J = 7.5 Hz, 2H), 8.08 (d, J = 7.9 Hz, 2H), 7.65 (d, J = 7.7 Hz, 1H), 7.38 (s, 1H), 7.27-7.20 (m, 1H), 7.04-6.95 (m, 2H), 6.84 (s, 1H), 6.60 (d, J = 8.0 Hz, 1H), 3.30-3.25 (m, 4H), 3.10 (s, 3H), 2.63-2.58 (m, 4H), 2.38 (s, 3H)。质谱:MS = 463 (MH)+。
参考文献:
[1] Journal of Medicinal Chemistry, 2012, vol. 55, # 11, p. 5243 - 5254
[2] Organic Process Research and Development, 2016, vol. 20, # 12, p. 2085 - 2091
[3] Patent: EP2648728, 2016, B1. Location in patent: Paragraph 0047