1260169-02-5
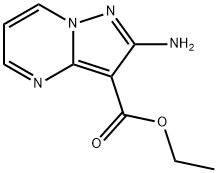 1260169-02-5 结构式
1260169-02-5 结构式
基本信息
2-Aminopyrazolo[1,5-a]pyrimidine-3-carboxylic acid ethyl ester
Pyrazolo[1,5-a]pyrimidine-3-carboxylic acid, 2-amino-, ethyl ester
物理化学性质
制备方法

102-52-3

6825-71-4
![2-氨基吡唑并[1,5-A]嘧啶-3-羧酸乙酯](/CAS/20180808/GIF/1260169-02-5.gif)
1260169-02-5
以3,5-二氨基-1H-吡唑-4-羧酸乙酯(5.00g,29.38mmol)和1,1,3,3-四甲氧基丙烷(14.50mL,88.15mmol)为原料,在DMF(80mL)中反应。加入AcOH(0.34mL,5.88mmol)作为催化剂。将反应混合物在100℃下搅拌14小时。反应完成后,通过真空浓缩去除溶剂。将残余物在DCM(50mL)和水(50mL)之间分配,分离有机相。水相用DCM(100mL×3)萃取。合并有机相,用盐水(100mL)洗涤,经无水Na2SO4干燥,过滤并真空浓缩。通过硅胶柱色谱法(洗脱剂:NH3在MeOH中的溶液(7M)/DCM(v/v)=1/100)纯化残余物,得到2-氨基吡唑并[1,5-a]嘧啶-3-羧酸乙酯,为浅黄色固体(3.52g,收率58.1%)。MS(ESI,阳离子)m/z:207.1 [M+H]+;1H NMR(400MHz,CDCl3):δ(ppm)8.60(dd,J=4.40Hz,1.76Hz,1H),8.46(dd,J=6.76Hz,1.76Hz,1H),6.86(dd,J=6.72Hz,4.40Hz,1H),4.50(q,J=7.08Hz,2H),1.47(t,J=7.08Hz,3H)。
参考文献:
[1] Patent: WO2015/73267, 2015, A1. Location in patent: Paragraph 318
[2] Patent: CN104650092, 2017, B. Location in patent: Paragraph 0463-0464; 0473-0474
[3] Patent: WO2011/3065, 2011, A2. Location in patent: Page/Page column 110-111
[4] Patent: US2015/336962, 2015, A1. Location in patent: Paragraph 0478
[5] Patent: WO2017/48702, 2017, A1. Location in patent: Paragraph 001169; 001170; 001172