1266322-58-0
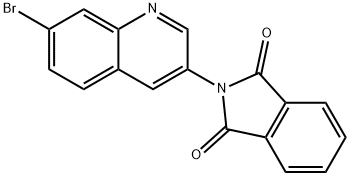
 1266322-58-0 结构式
1266322-58-0 结构式
制备方法
1375108-38-5

1266322-58-0
b) 7-溴喹啉-3-胺的合成:将2-(7-溴喹啉-3-基)异吲哚啉-1,3-二酮(10g,28.3mmol)悬浮于乙醇(200mL)中,加入肼(1.777mL,56.6mmol),随后加热回流1小时。反应完成后,使混合物冷却至室温,收集沉淀并用少量乙醇洗涤。将滤液浓缩至干,得到灰色固体。将所得固体溶解于温乙醇中,并吸附于硅胶上。通过硅胶柱色谱法(洗脱剂:50-100%乙酸乙酯/己烷梯度)纯化,得到目标化合物7-溴喹啉-3-胺(3.5g,收率56%)。1H NMR(400MHz,DMSO-d6)δ ppm:5.83(s,2H),7.14(d,J = 2.53Hz,1H),7.49(dd,J = 8.84, 2.02Hz,1H),7.54-7.65(m,1H),7.94(d,J = 1.77Hz,1H),8.46(d,J = 2.78Hz,1H)。
参考文献:
[1] Patent: WO2017/32840, 2017, A1. Location in patent: Page/Page column 265; 266
[2] Patent: WO2013/28447, 2013, A1. Location in patent: Page/Page column 117
[3] Patent: WO2013/52716, 2013, A1. Location in patent: Page/Page column 63
[4] Patent: WO2013/177253, 2013, A2. Location in patent: Page/Page column 64
[5] Patent: WO2014/8223, 2014, A2. Location in patent: Page/Page column 71-72
