127423-61-4
 127423-61-4 结构式
127423-61-4 结构式
基本信息
(R)-1-BOC-3-甲磺酰氧基吡咯烷
(R)-3-[(甲基磺酰基)氧基]吡咯烷-1-羧酸叔丁酯
(R)-叔丁基3-((甲基磺酰基)氧基)吡咯烷-1-羧酸酯
(R)-1-Boc-3-[(methylsulfonyl)oxy]pyrrolidine
(3R)-N-(tert-Butoxycarbonyl)-3-(methylsulfonyloxy)pyrrolidine
tert-Butyl (R)-3-(methylsulfonyloxy)pyrrolidine-1-carboxylate
tert-Butyl (R)-3-[(methylsulfonyl)oxy]pyrrolidine-1-carboxylate
tert-Butyl (3R)-3-[(methylsulfonyl)oxy]pyrrolidine-1-carboxylate
(R)-3-[(Methylsulfonyl)oxy]pyrrolidine-1-carboxylic acid tert-butyl ester
1-Pyrrolidinecarboxylic acid, 3-[(methylsulfonyl)oxy]-, 1,1-dimethylethylester, (3R)-
物理化学性质
制备方法

83220-73-9
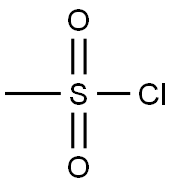
124-63-0

127423-61-4
一般步骤: 步骤1: (R)-1-(叔丁氧基羰基)吡咯烷-3-基甲磺酸酯的合成。在冰水浴中冷却(R)-3-羟基吡咯烷-1-羧酸叔丁酯(1.0g,5.3mmol)和三乙胺(2.77mL,20mmol)的二氯甲烷(20mL)溶液。向该溶液中滴加稀释于二氯甲烷(10mL)中的甲磺酰氯(1.15mL,15mmol)。反应混合物在室温下搅拌12小时。反应完成后,加入水,用二氯甲烷(3×50mL)萃取有机相。合并的有机相经干燥后,通过硅胶色谱法(50%乙酸乙酯:己烷至100%乙酸乙酯梯度)纯化,得到(R)-1-(叔丁氧基羰基)吡咯烷-3-基甲磺酸酯(1.53g,棕色油状物,收率100%)。
参考文献:
[1] Patent: WO2010/45542, 2010, A2. Location in patent: Page/Page column 73
[2] Patent: WO2012/62783, 2012, A1. Location in patent: Page/Page column 75
[3] Patent: WO2017/12576, 2017, A1. Location in patent: Page/Page column 187
[4] Patent: WO2018/71454, 2018, A1. Location in patent: Paragraph 001372; 001373; 001374
[5] Journal of Enzyme Inhibition and Medicinal Chemistry, 2018, vol. 33, # 1, p. 1460 - 1471
