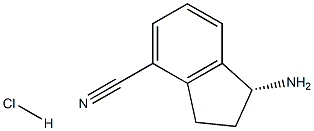
1306763-30-3
 1306763-30-3 结构式
1306763-30-3 结构式
基本信息
(S)-N-BOC-1-氨基-4-氰基-2,3-二氢茚
(R)-(4-氰基-2,3-二氢-1H-茚-1-基)氨基甲酸叔丁酯
(S)-tert-butyl (4-cyano-2,3-dihydro-1H-inden-1-yl)carbamate
TERT-BUTYL N-[(1R)-4-CYANO-2,3-DIHYDRO-1H-INDEN-1-YL]CARBAMATE
Carbamic acid, N-[(1R)-4-cyano-2,3-dihydro-1H-inden-1-yl]-, 1,1-dimethylethyl ester
制备方法

24424-99-5
1306763-29-0

1306763-30-3
在0℃下,将(R)-1-氨基-2,3-二氢-1H-茚-4-甲腈盐酸盐(INT-50,11.6g,59.6mmol)溶于二氯甲烷(DCM,100mL)中,加入三乙胺(TEA,12.0mL,131.0mmol)。随后,向反应体系中缓慢加入二碳酸二叔丁酯(Boc酸酐,14.3g,65.6mmol)的DCM(30mL)溶液。反应混合物在室温下搅拌1.5小时。反应完成后,用盐水洗涤反应混合物,有机层用无水硫酸镁(MgSO4)干燥并过滤。向滤液中加入额外的DCM至总体积为250mL,并加入活性炭(Norit,4.5g)。将混合物回流15分钟后,趁热通过硅藻土/二氧化硅垫过滤。浓缩滤液,通过乙酸乙酯(EA,50mL)和己烷(150mL)重结晶,得到12.93g(产率84%)的(R)-(4-氰基-2,3-二氢-1H-茚-1-基)氨基甲酸叔丁酯(INT-52),为灰白色固体。LCMS-ESI(m/z): C15H18N2O2的计算值为258.3;实测值为281.1 [M+Na]+,保留时间(tR)=3.45分钟。元素分析结果(C15H18N2O2): 计算值C=69.74%,H=7.02%,N=10.84%;实测值C=69.98%,H=7.14%,N=10.89%。1H NMR(400MHz,CDCl3)δ: 7.64-7.49(m,2H),7.34(dt,J=7.7,3.8Hz,1H),5.36-5.20(m,1H),4.78(d,J=6.8Hz,1H),3.20(ddd,J=16.9,8.9,3.3Hz,1H),3.02(dt,J=25.4,8.4Hz,1H),2.82-2.53(m,1H),1.88(dq,J=13.2,8.6Hz,1H),1.55-1.44(m,9H)。13C NMR(101MHz,DMSO)δ: 155.52,146.68,146.32,130.89,128.70,127.63,117.51,107.76,77.98,55.09,31.88,29.11,28.19。手性HPLC分析:使用2.5%乙醇的己烷溶液作为洗脱剂,(R)-4-氰基-2,3-二氢-1H-茚-1-基氨基甲酸叔丁酯的对映体过量(ee)>99.9%,保留时间(tR)=19.36分钟。
参考文献:
[1] Patent: WO2011/60389, 2011, A1. Location in patent: Page/Page column 92-93
[2] Patent: WO2015/66515, 2015, A1. Location in patent: Page/Page column 78-79
[3] Patent: WO2018/64356, 2018, A1. Location in patent: Page/Page column 57
