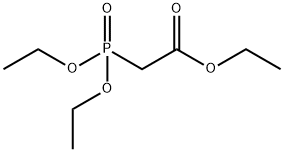
135716-08-4
 135716-08-4 结构式
135716-08-4 结构式
基本信息
2-(1-BOC-4-亚哌啶基)乙酸乙酯
1-BOC-4-(2-乙氧基-2-氧代亚乙基)哌啶
4-(2-乙氧基-2-氧代亚乙基)-1-哌啶羧酸叔丁酯
4-(2-乙氧基-2-氧代亚乙基)哌啶-1-甲酸叔丁酯
叔丁基4-(2-乙氧基-2-氧代亚乙基)哌啶-1-羧酸
4-(2-乙氧基-2-氧代-亚乙基)哌啶-1-羧酸叔-丁酯
Ethyl 2-(1-Boc-4-piperidylidene)acetate
tert-Butyl 4-(2-ethoxy-2-oxoethylidene)
Ethyl (1-Boc-piperidin-4-ylidene)acetate
_x000D_Ethyl 2-(1-Boc-4-piperidylidene)acetate
Ethyl [1-(tert-Butoxycarbonyl)piperidin-4-ylidene]acetate
1-piperidinecarboxylic acid, 4-(2-ethoxy-2-oxoethylidene)-
4-(Ethoxycarbonylmethylene)-1-(tert-butoxycarbonyl)piperidine
tert-Butyl 4-(2-ethoxy-2-oxoethylidene)piperidin-1-carboxylate
tert-Butyl 4-(2-ethoxy-2-oxoethylidene)-1-piperidinecarboxylate
物理化学性质
制备方法
867-13-0

79099-07-3

135716-08-4
以磷酰基乙酸三乙酯和N-叔丁氧羰基-4-哌啶酮为原料合成4-(2-乙氧基-2-氧代-亚基)哌啶-1-羧酸叔丁酯的一般步骤如下:向乙基二乙基磷酰乙酸乙酯(28.3 g)的四氢呋喃(200 mL)溶液中加入60%氢化钠(4.82 g)。在冰浴冷却下搅拌混合物30分钟,随后缓慢滴加N-叔丁氧羰基-4-哌啶酮(20 g)的四氢呋喃(200 mL)溶液。将反应混合物在室温下持续搅拌22小时。反应完成后,加入水(200 mL)终止反应,并用乙酸乙酯进行萃取。合并有机相,用饱和氯化钠水溶液洗涤,随后用无水硫酸镁干燥。减压浓缩后,通过硅胶柱色谱法纯化粗产物,使用己烷/乙酸乙酯(6:1)作为洗脱剂,最终得到4-(2-乙氧基-2-氧代-亚基)哌啶-1-羧酸叔丁酯(27.3 g,收率100%),为无色粉末。产物经1H NMR(CDCl3)表征:δ 1.28(3H,t,J = 7.4 Hz),1.47(9H,s),2.24-2.33(2H,m),2.90-2.98(2H,m),3.43-3.55(4H,m),4.16(2H,q,J = 7.4 Hz),5.70-5.73(1H,m)。
参考文献:
[1] Heterocycles, 2001, vol. 54, # 2, p. 747 - 755
[2] Patent: EP1498125, 2005, A1. Location in patent: Page/Page column 84-85
[3] Patent: WO2018/152329, 2018, A1. Location in patent: Page/Page column 48
[4] Patent: CN102952072, 2016, B. Location in patent: Paragraph 0109
[5] Chinese Chemical Letters, 2012, vol. 23, # 6, p. 707 - 710
