136834-21-4
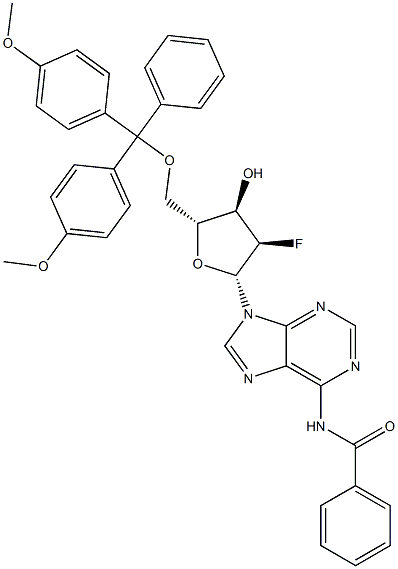 136834-21-4 结构式
136834-21-4 结构式
基本信息
N6-苯甲酰基-2-脱氧-5-O-DMT-2-氟腺苷酸
5'-O-(4,4'-二甲氧基三苯甲基)-N6-苯甲酰基-2'-氟脱氧腺苷
N-苯甲酰基-5'-O-[双(4-甲氧基苯基)苯甲基]-2'-脱氧-2'-氟腺苷
N6-苯甲酰基-2'-脱氧-2'-氟-5'-O-(4,4'-二甲氧基三苯甲基)腺苷
N-苯甲酰基-5'-O-[二(4-甲氧基苯基)苯基甲基]-2'-脱氧-2'-氟腺苷
N-(9-((2R,3R,4R,5R)-5-((双(4-甲氧基苯基)(苯基)甲氧基)甲基)-3-氟-4-羟基四氢呋喃-2-基)-9H-嘌呤-6-基)苯甲酰胺
N-(9-((2R,3R,4R,5R)-5-((BIS(4-METHOXYPHENYL)(PHENYL)METHOXY)METHYL)-3-FLUORO-4-HYDROXYTETRAHYDROFURAN-2-YL)-9H-PURIN-6-YL)BENZAMIDE
5'-DMT-2'-F-Bz-dA
5'-O-DMT-N6-Bz-2'-F-dA
5'-O-DMT-2'-F-Benzoyl-Deoxyadenosine
5'-O-DMT-2'-Fluoro-N6-Benzoyl-2'-deoxyadenosine
N6-Benzoyl-5'-O-DMT-2'-fluoro-2'-deoxyadenosine
5'-O-DMT-N6-BENZOYL-2'-FLUORO-2'-DEOXYADENOSINE
N6-benzoyl-2'-deoxy-2'-fluoro-5'-O-(4,4'-dimethoxytrityl)adenosine
N-Benzoyl-5'-O-[bis(4-methoxyphenyl)phenylmethyl]-2'-deoxy-2'-fluoroadenosine
Adenosine, N-benzoyl-5'-O-[bis(4-Methoxyphenyl)phenylMethyl]-2'-deoxy-2'-fluoro-
物理化学性质
制备方法

40615-36-9

136834-20-3

136834-21-4
将N-(9-((2R,3R,4R,5R)-3-氟-4-羟基-5-(羟甲基)四氢呋喃-2-基)-9H-嘌呤-6-基)苯甲酰胺(570g,1.53mol)溶解于吡啶(2.85L,35.2mol)中。将反应混合物冷却至2.6℃,随后加入4,4'-双甲氧基三苯甲基氯(543g,1.60mol)。在0至5℃下搅拌反应混合物2小时,然后缓慢升温至室温。通过LC/MS监测反应进程,确认反应完全后,将混合物冷却至5℃以下,缓慢加入甲醇(124ml,3.05mol)淬灭反应,持续搅拌15分钟。随后,将反应混合物与甲苯(2.00L)共蒸发浓缩,再用乙酸乙酯(2.85L)和正庚烷(2.85L)的混合溶剂稀释。用9%的饱和碳酸氢钠水溶液(2.0L)洗涤有机层,再加入乙酸乙酯(2.85L)以确保粗产物完全溶解。搅拌5分钟后,分离有机层和水层。有机层用水(2.0L)洗涤后,开始有固体缓慢析出。分离水层后,将有机层浓缩至约原体积的1/50。粗产物用正庚烷(2.00L)和甲苯(0.50L)的混合溶剂浆化,搅拌15分钟后,通过真空过滤收集浅黄色固体。滤饼依次用(1)正庚烷(0.60L)和甲苯(0.30L)的混合溶剂及(2)正庚烷(3.00L)洗涤。固体在室温下干燥30分钟后,转移至托盘,于50℃真空烘箱中干燥过夜,得到目标化合物N-(9-((2R,3R,4R,5R)-5-((双(4-甲氧基苯基)(苯基)甲氧基)甲基)-3-氟-4-羟基四氢呋喃-2-基)-9H-嘌呤-6-基)苯甲酰胺(996.7g,1.47mol,产率97%)。NMR数据确认产物结构。
参考文献:
[1] Patent: WO2018/152450, 2018, A1. Location in patent: Page/Page column 61; 62
[2] Bioorganic and Medicinal Chemistry Letters, 2010, vol. 20, # 15, p. 4539 - 4543
[3] Patent: WO2018/156625, 2018, A1. Location in patent: Paragraph 0290; 0291
[4] Journal of Medicinal Chemistry, 1993, vol. 36, # 7, p. 831 - 841
[5] Tetrahedron Letters, 1998, vol. 39, # 13, p. 1657 - 1660
