137130-65-5
 137130-65-5 结构式
137130-65-5 结构式
基本信息
叔丁氧羰基-D-天冬氨酸 1-甲酯
N-[叔丁氧羰基]-D-天冬氨酸甲酯
N-BOC-D-天冬氨酸1-甲酯,97%
(Tert-Butoxy)Carbonyl D-Asp-OMe
N-Boc-D-aspartic acid 1-methyl ester
N-Boc-D-asparticacid1-methylester,97%
α-methyl N-(tert-butoxycarbonyl)aspartate
(R)-3-((tert-butoxycarbonyl)amino)-4-methoxy-4-oxobutanoic acid
N-[(1,1-Dimethylethoxy)carbonyl]-D-aspartic acid 1-methyl ester
(3R)-3-{[(tert-butoxy)carbonyl]amino}-4-methoxy-4-oxobutanoic acid
D-Aspartic acid, N-[(1,1-dimethylethoxy)carbonyl]-, 1-methyl ester
物理化学性质
制备方法
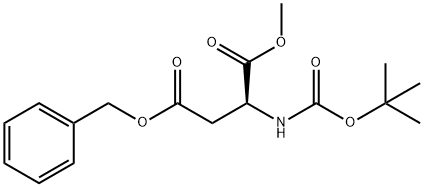
80963-12-8
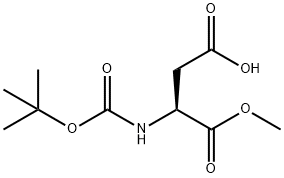
98045-03-5
以Boc-L-天冬氨酰(OBn)(OMe)14(5.88g,17.43mmol)为原料,将其溶解于50mL圆底烧瓶中的甲醇(20mL)中。在氮气保护下,向反应体系中加入10%钯碳催化剂。随后,用氮气置换反应容器内的气体三次,再用氢气置换三次。将反应混合物在氢气氛围及常压下搅拌6小时。反应完成后,通过过滤移除催化剂,并将滤液浓缩,得到目标产物(S)-3-((叔丁氧羰基)氨基)-4-甲氧基-4-氧代丁酸(化合物15),为无色胶状液体(4.1克,收率95%)。产物的旋光度[α]21D = 34(c 0.1,CHCl3)。1H NMR(CDCl3, 400MHz)δ: 1.43(s, 9H), 2.83-2.86(d, 1H, J = 16.92Hz), 3.01-3.05(d, 1H, J = 16.68Hz), 3.74(s, 3H), 4.57(s, 1H), 5.55-5.57(d, 1H, J = 8.12Hz), 10.45(s, 1H)。13C NMR(CDCl3, 100MHz)δ: 28.3, 36.6, 49.7, 52.8, 80.5, 155.5, 171.5, 176.0。IR(KBr)νmax: 3366, 2980, 1719, 1506, 1440, 1395, 1368, 1222, 1165, 1060, 1008, 864, 764, 587 cm-1。
参考文献:
[1] Organic and Biomolecular Chemistry, 2007, vol. 5, # 9, p. 1459 - 1465
[2] Tetrahedron Letters, 2014, vol. 55, # 14, p. 2274 - 2276
[3] Bioorganic and Medicinal Chemistry Letters, 2005, vol. 15, # 1, p. 15 - 20
[4] Bioorganic and Medicinal Chemistry Letters, 2010, vol. 20, # 2, p. 586 - 590
[5] Carbohydrate Research, 2013, vol. 382, p. 36 - 42
