1374639-77-6
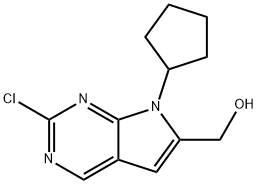 1374639-77-6 结构式
1374639-77-6 结构式
基本信息
LEE011中间体
LEE011中间体2
瑞柏司可里布中间体2
2-氯-7-环戊基-7H-吡咯并[2,3-D]嘧啶-6-甲醇
2-氯-6-(羟甲基)-7-环戊基-7H-吡咯并[2,3-D]嘧啶
Ribociclib Intermediate 2
Ribociclib Chloro Hydroxymethyl IMP
Ribociclib Chloro Hydroxymethyl Impurity
(2-chloro-7-cyclopentyl-7H-pyrrolo[2,3-d]pyriMidin
2-Chloro-7-cyclopentyl-7H-pyrrolo[2,3-d]pyriMidine-6-Methanol
(2-chloro-7-cyclopentyl-7H-pyrrolo[2,3-d]pyrimidin-6-yl)meth...
7H-Pyrrolo[2,3-d]pyrimidine-6-methanol, 2-chloro-7-cyclopentyl-
(2-chloro-7-cyclopentyl-7H-pyrrolo[2,3-d]pyriMidin-6-yl)Methanol
2-Chloro-7-cyclopentyl-6-(hydroxymethyl)-7H-pyrrolo[2,3-d]pyrimidine
物理化学性质
制备方法
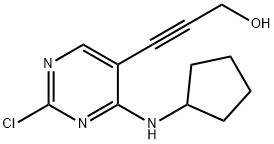
1374639-76-5
![2-氯-7-环戊基-7H-吡咯并[2,3-D]嘧啶-6-甲醇](/CAS/20150408/GIF/1374639-77-6.gif)
1374639-77-6
以3-(2-氯-4-(环戊基氨基)嘧啶-5-基)丙-2-炔-1-醇为原料合成2-氯-7-环戊基-7H-吡咯并[2,3-D]嘧啶-6-甲醇的一般步骤:在氮气保护下,将化合物(3)(5g,19.9mmol)溶于THF(50mL)中,于29℃下搅拌。向此溶液中缓慢加入1.0M四丁基氟化铵的THF溶液(45mL),随后将反应混合物加热至60℃,反应进程通过TLC监测。反应完成后,将混合物在减压下浓缩,残余物溶于2-丙醇(10mL)中,并于50℃下搅拌至完全溶解。将所得澄清溶液冷却至29℃,缓慢加入水(75mL),继续搅拌4小时。析出的固体经过滤收集,用水洗涤,并于50℃下真空干燥,得到化合物(4)为棕色固体,产率68%,熔点173-175℃。1H NMR(400MHz,DMSO-d6)δ:1.64-1.65(m,2H,-CH2),1.98-2.03(m,4H,-CH2),2.22-2.29(m,2H,-CH2),4.67(d,2H,J=5.12Hz,-CH2OH),4.87-4.89(m,1H,-NH-CH-),5.52(s,1H,-CH2OH),6.55(s,1H,Ar-H),8.82(s,1H,Ar-H)。13C NMR(75.46MHz,DMSO-d6)δ:152.3,151.5,150.9,144.7,118.21,98.5,56.4,52.3,30.7,24.9。ESI-HRMS(m/z):计算值C12H14ClN3O的[M+]为251.7129,实测值251.8001。
参考文献:
[1] Combinatorial Chemistry and High Throughput Screening, 2017, vol. 20, # 8, p. 703 - 712
[2] Patent: US2012/115878, 2012, A1. Location in patent: Page/Page column 7
[3] Patent: WO2018/51280, 2018, A1. Location in patent: Page/Page column 20
