145681-01-2
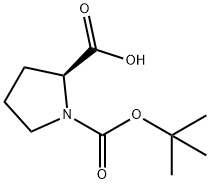
 145681-01-2 结构式
145681-01-2 结构式
基本信息
Boc-DL-proline Methyl ester
Methyl 1-Boc-2-pyrrolidinecarboxylate
Methyl N-Boc-Pyrrolidine-2-Carboxylate
1-Boc-2-pyrrolidinecarboxylic acid Methyl ester
1-O-tert-butyl 2-O-methyl pyrrolidine-1,2-dicarboxylate
1,2-Pyrrolidinedicarboxylic acid, 1-(1,1-diMethylethyl) 2-Methyl ester
物理化学性质
制备方法
15761-39-4
74-88-4

145681-01-2
以Boc-L-脯氨酸和碘甲烷为原料合成1-Boc-2-吡咯烷甲酸甲酯的一般步骤如下:将碳酸钾(K2CO3,1.1 kg,8.0 mol)和碘甲烷(CH3I,659 g,4.65 mol)加入到1-[(2-甲基丙烷-2-基)氧基羰基]吡咯烷-2-羧酸(500 g,2.32 mol)的二甲基甲酰胺(DMF,2.5 L)溶液中。在室温下搅拌反应混合物12小时,随后进行过滤。将滤液在真空下浓缩。将残余物溶解于乙酸乙酯(EtOAc,2 L)中,依次用水(2×1 L)和盐水(1 L)洗涤,用硫酸镁(MgSO4)干燥,最后在真空下浓缩,得到1-Boc-2-吡咯烷甲酸甲酯(417.8 g,收率96%)为黄色油状物。1H NMR(CDCl3)数据如下:δ 1.37(9H,m),1.72-1.84(3H,m),2.15(1H,m),3.26-3.51(2H,m),3.65(3H,s),4.17(1H,m)。
参考文献:
[1] Patent: WO2013/14448, 2013, A1. Location in patent: Page/Page column 103
[2] Patent: WO2016/89977, 2016, A1. Location in patent: Paragraph 00137
[3] Patent: WO2014/28675, 2014, A1. Location in patent: Page/Page column 118
[4] Patent: WO2016/138532, 2016, A1. Location in patent: Paragraph 0376
[5] Tetrahedron Asymmetry, 2003, vol. 14, # 10, p. 1323 - 1333
