146504-07-6
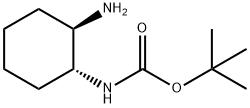 146504-07-6 结构式
146504-07-6 结构式
基本信息
左旋-1R,2R-N-BOC-环己二胺
1R,2R-N-BOC-环己二胺 5G
(1R,2R)-N-BOC-1,2-环己二胺
(1R)-反式-N-BOC-1,2-二氨基环己烷
[(1R,2R)-2-氨基环己基]氨基甲酸叔丁酯
(1R,2R)-反式-N-BOC-1,2-环己二胺
(1R,2R)-N1-叔丁氧羰基-1,2-环己二胺
(1R,2R)-1-(BOC-氨基)-2-氨基环己烷
N-叔丁氧羧基-反式-1,2-环己二胺(1R,2R)
N-Boc-1R,2R-Cyclohexanediamine
(1R,2R)-N-Boc-1,2-cyclohexanediamine
(1R,2R)-N1-Boc-1,2-cyclohexanediamine
(1R)-trans-N-Boc-1,2-diaminocyclohexane
(1R,2R)-2-Amino-1-(Boc-amino)cyclohexane
(1R,2R)-1-AMINO-2-(BOC-AMINO)-CYCLOHEXANE
(1R,2R)-trans-N-Boc-1,2-Cyclohexanediamine
Trans (1R,2R)-1N-Boc-cyclohexane-1,2-diamine
N-tert-Butoxycarbonyl-R,R-1,2-diaminocyclohexane
物理化学性质
安全数据
制备方法
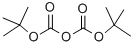
24424-99-5

20439-47-8

146504-07-6
以二碳酸二叔丁酯和左旋-反式-1,2-环己二胺为原料合成(1R,2R)-N-Boc-1,2-环己二胺的一般步骤:将(R,R)-环己烷二胺(5.70g,50.0mmol)溶解于1,4-二恶烷(110.0mL)中。在室温搅拌下,缓慢滴加Boc-酐(1.09g,5.0mmol)的1,4-二恶烷(35.0mL)溶液。滴加完毕后,继续搅拌反应5小时。反应完成后,通过旋转蒸发除去溶剂,将所得残余物溶于水(55mL)中。过滤除去不溶物,滤液用二氯甲烷(3×55mL)萃取。合并有机相,用水洗涤,分离有机层,用无水硫酸镁干燥,过滤后通过旋转蒸发浓缩。最后,通过硅胶柱色谱法纯化,得到黄色固体产物(1R,2R)-N-Boc-1,2-环己二胺(1.00g,收率94%)。
参考文献:
[1] Journal of the Chemical Society, Dalton Transactions, 2001, # 14, p. 2188 - 2198
[2] Patent: CN105017172, 2017, B. Location in patent: Paragraph 0043; 0044
[3] European Journal of Organic Chemistry, 2018, vol. 2018, # 1, p. 99 - 103
[4] Journal of the American Chemical Society, 2011, vol. 133, # 37, p. 14704 - 14709
[5] Organic and Biomolecular Chemistry, 2018, vol. 16, # 35, p. 6423 - 6429
