1533519-85-5
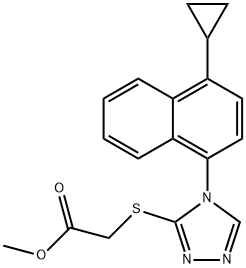 1533519-85-5 结构式
1533519-85-5 结构式
基本信息
雷西那德杂质11
雷西纳德中间体1
蕾西纳德原料及其中间体
LESINURAD杂质9
LESINURAD中间体4
LESINURAD中间体1
4-三唑-3-基]硫基]乙酸甲酯
2-[[4-(4-环丙基萘-1-基)-4H-1
2-(4-(4-环丙基萘-1-基)-4H-1,2,4-噻唑-3-硫基)乙酸甲酯
LSEINURAD INT
Lesinurad INT4
Lesinurad internate 4
Intermediate of lesinurad
4-triazol-3-yl]thio]acetate
Pentanedioicacid,5-oxo-,dimethylester
Methyl 2-[[4-(4-cyclopropylnaphthalen-1-yl)-4H-1
Acetic acid, 2-((4-(4-cyclopropyl-1-naphthalenyl)-4H-1,2,4-t...
Methyl 2-[[4-(4-cyclopropylnaphthalen-1-yl)-4H-1,2,4-triazol-3-yl]thio]acetate
物理化学性质
制备方法

1533519-84-4

96-32-2
![2-[[4-(4-环丙基萘-1-基)-4H-1,2,4-三唑-3-基]硫基]乙酸甲酯](/CAS/20150408/GIF/1533519-85-5.gif)
1533519-85-5
在装有磁力搅拌器和温度计的100 mL三颈圆底烧瓶中,加入25 mL N,N-二甲基甲酰胺(DMF)和中间体(III)(2.67 g,1-环丙基-4-异硫代氰酰基萘)。在室温下搅拌至完全溶解后,依次加入碳酸钾(1.45 g)和溴乙酸甲酯(1.61 g)。控制反应温度在25℃,缓慢滴加完毕后,继续搅拌反应3小时。反应完成后,向反应混合物中加入50 mL水稀释,随后用乙酸乙酯(50 mL × 3)进行萃取。合并有机相,用旋转蒸发仪浓缩除去乙酸乙酯。向浓缩后的残余物中加入50 mL甲醇,加热至回流使晶体溶解,随后缓慢冷却至室温进行重结晶。通过抽滤收集晶体,并用少量冷甲醇洗涤,真空干燥后得到中间体(IV)(2-((4-(4-环丙基萘-1-基)-4H-1,2,4-三唑-3-基)硫代)乙酸甲酯)3.22 g,产率99.5%。
参考文献:
[1] Patent: CN105906576, 2016, A. Location in patent: Paragraph 0070; 0071; 0083; 0084
[2] Patent: WO2014/8295, 2014, A1. Location in patent: Paragraph 0254-0262
[3] Patent: CN105399694, 2016, A. Location in patent: Paragraph 0085; 0086; 0087; 0088
[4] Patent: CN107098866, 2017, A. Location in patent: Paragraph 0079; 0080; 0081; 0082; 0083
