153773-82-1
 153773-82-1 结构式
153773-82-1 结构式
基本信息
厄他培南粗品
厄他培南钠盐
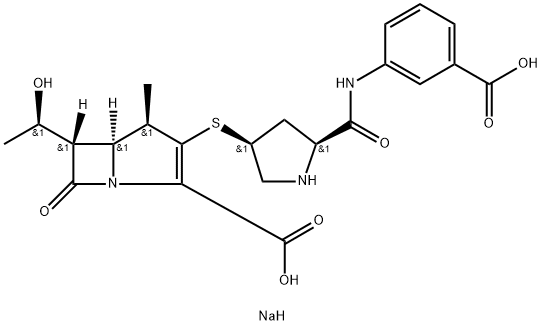
(4R,5S,6S)-3-[[(3S,5S)-5-[[(3-羧基苯基)氨基甲酰基]吡咯烷-3-基]硫-6-(1-羟基乙基)-4-甲基-7-氧代-1-氮杂双环[3.2.0]庚-2-烯-2-甲酸钠盐
(4R,5R,6S)-3-[(3S,5S)-5-[(3-羧基苯基)氨基甲酰基]吡咯烷-3-基]硫-6-(1-羟乙基)-4-甲基-7-氧代-1-氮杂双环[3.2.0]庚-2-烯-2-甲酸钠盐/厄他培南钠盐/尔他培南钠盐/呃他培南钠盐
3-(((3S
L 749345
EtapeneM
Etapenem sodium
ErtapeneM Crude
ErtapeneM MonosodiuM
Ertapenem Impurity 16
Ertapenem sodium, >=97%
He Seoul sodium imipenem
物理化学性质
制备方法
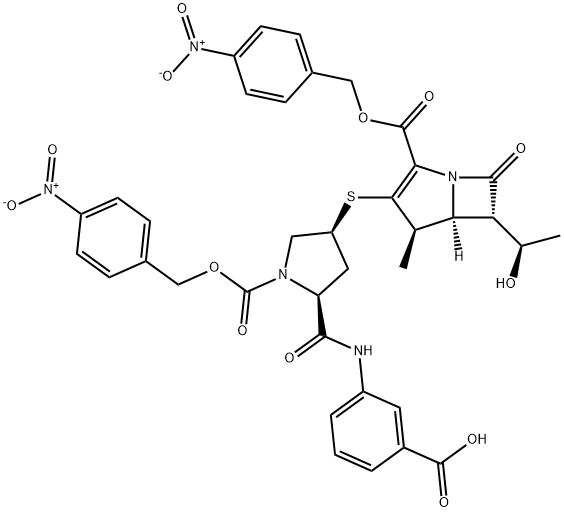
202467-70-7

153773-82-1
以化合物(CAS: 202467-70-7)为原料,按照CN102731508A中所述的方法,合成(4R,5S,6S)-3-(((3S,5S)-5-((3-羧基苯基)氨基甲酰基)吡咯烷-3-基)硫基)-6-((R)-1-羟乙基)-4-甲基-7-氧代-1-氮杂双环[3.2.0]庚烷-2-烯-2-甲酸单钠盐的一般步骤如下:在750 mL乙酸乙酯和500 mL水的混合溶剂中,加入实施例1制备的双重保护的厄他培南晶体形式(10 g,0.017 mol)。用5%氢氧化钠水溶液调节pH至7.1-7.2,随后加入15 g钯碳催化剂。在氢气压力为20.0-30.0 MPa的条件下氢化反应2小时。反应完成后,过滤去除催化剂,滤液用二氯甲烷(100 mL×2)萃取,分离水层。水层通过大孔吸附树脂进行吸附,随后用2 L去离子水洗涤树脂。接着,用含0.05 M碳酸氢钠的丙酮溶液洗脱,洗脱液酸化至pH 5.5后,采用纳滤法浓缩至50 mL。向浓缩液中加入100 g甲醇与正丙醇的混合溶剂(W/W = 1:1),进一步浓缩促进结晶。结晶过程温度控制在10-20℃,结晶完成后过滤收集产物。产物在<0.09 MPa的真空条件下干燥3小时,最终得到厄他培南单钠盐3.4 g,收率为40.2%,HPLC纯度为99.2%。
参考文献:
[1] Patent: CN104788452, 2017, B. Location in patent: Paragraph 0067; 0068
[2] Patent: WO2013/121279, 2013, A2. Location in patent: Page/Page column 15
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | HY-13625 | 厄他培南钠 Ertapenem sodium | 153773-82-1 | 1 mg | 187元 |
| 2025/12/22 | HY-13625 | 厄他培南钠 Ertapenem sodium | 153773-82-1 | 5 mg | 375元 |
| 2025/12/22 | HY-13625 | 厄他培南钠 Ertapenem sodium | 153773-82-1 | 10 mg | 600元 |
常见问题列表
Ertapenem sodium inhibits
Streptococcus pneumoniae
with MIC
50
s values < 1mg/L.
Ertapenem sodium exhibits a bactericidal mode of action as shown by time-killing curves and exhibits a short postantibiotic effect (PAE) of 1.4-2.6 h against the Gram-positive strains but no PAE against Gram-negative strains.
Ertapenem sodium (50 mg/kg; s.c.; q6h; for 24 hours) shows substantial bactericidal activity against
S. pneumoniae
in the murine thigh infection model.
Ertapenem sodium exhibits an AUC
0-24
of 586 mg•h/L, C
max
of 140 mg/L following subcutaneous injection (mice 50 mg/kg).
| Animal Model: | Specific-pathogen-free female ICR mice (~25 g), thigh infection model |
| Dosage: | 50 mg/kg |
| Administration: | Subcutaneous injection, q6h, for 24 hours |
| Result: | Showed substantial bactericidal activities. |
| Animal Model: | Specific-pathogen-free female ICR mice (~25 g), thigh infection model |
| Dosage: | 20 mg/kg, 50 mg/kg, 100 mg/kg (Pharmacokinetic Analysis) |
| Administration: | Subcutaneous injection |
| Result: | AUC 0-24 (586 mg•h/L), C max (140 mg/L) at 50 mg/kg q6h dosing regimen. |
